Service hotline
+86 18518316054
 Current location : Home page > Resources > Papers > In situ synthesis of In2S3@MIL-125(Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis
Current location : Home page > Resources > Papers > In situ synthesis of In2S3@MIL-125(Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis
Hou Wanga,b , Xingzhong Yuana,b,c* , Yan Wud , Guangming Zenga,b , Haoran Donga,b , Xiaohong Chenc , Lijian Lenga,b , Zhibin Wua,b, Lijuan Penge
aCollege of Environmental Science and Engineering, Hunan University, Changsha 410082, PR China
bKey Laboratory of Environment Biology and Pollution Control, Hunan University, Ministry of Education, Changsha 410082, PR China
cCollaborative Innovation Center of Resource–Conserving & Environment-friendly Society and Ecological Civilization, Changsha 410083, PR China
dCollege of Environment and Energy, South China University of Technology, Guangzhou 510006, PR China
eGuangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou, 510006, PR China
Graphical Abstract
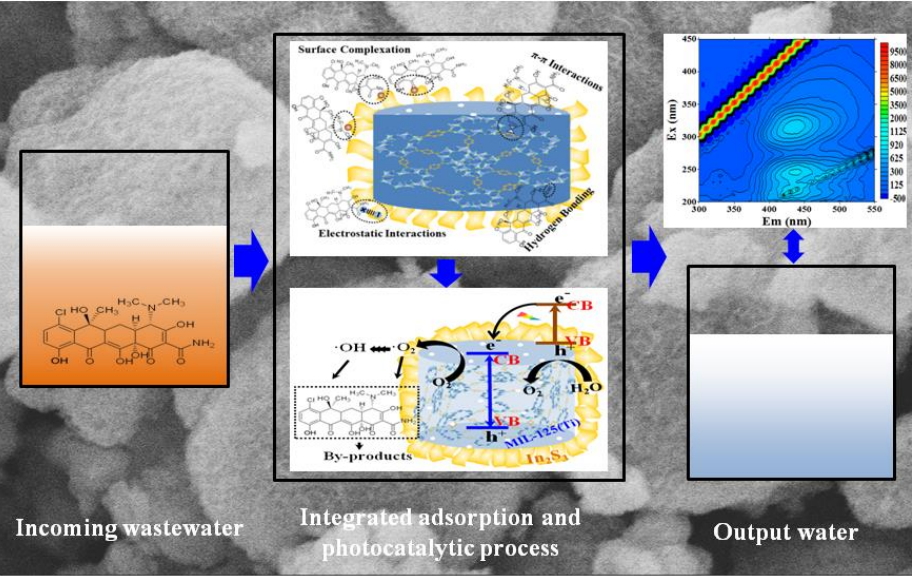
Abstract
Metal-organic frameworks (MOFs) have been attracted considerable attention in the field of energy generation and environmental remediation. In this article, a novel core-shell In2S3@MIL-125(Ti) (MLS) photocatalytic adsorbent was successfully prepared by a facile solvothermal method. The as-obtained materials were characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, N2 adsorption-desorption isotherm, X-ray photoelectron spectroscopy, ultraviolet–visible diffuse reflection spectroscopy and zeta potentials. It is indicated that the hybrids consisted of MIL-125(Ti) as the core and three-dimensional In2S3 sheets network as the shell has high surface area, mesoporous structure, and improved electronegativity and visible-light absorption. The MLS exhibited excellent adsorption performance for the removal of tetracycline (TC) from water. The adsorption process is sensitive to the solution pH, ionic strength and initial TC concentration. The Langmuir isotherm and pseudo-second-order mode could well describe the adsorption process and adsorption kinetics. The adsorption mechanism is mainly responsible for surface complexation, π-π interactions, hydrogen bonding and electrostatic interactions. Further, in TC degradation experiments under visible light exposure in presence of core-shell MLS, the optimal additive content of MIL-125(Ti) in synthesis process was 0.1 g, and the corresponding photodegradation efficiency for TC was 63.3%, which was higher than that of pure In2S3 and pure MIL-125(Ti). The improved photocatalytic performance was mainly ascribed to the opened porous structure, effective transfer of photo-generated carriers, Ti3+ –Ti4+ intervalence electron transfer and the synergistic effect between MIL-125(Ti) and In2S3. The degradation by-products of TC molecules were monitored by three-dimensional excitation-emission matrix fluorescence spectroscopy. Parts of TC molecules were mineralized into CO2 and H2O. The core-shell MLS composites also revealed good performance for the removal of TC from real wastewater including medical wastewater, municipal wastewater and river water. Therefore, the novel hybrids may be used as promising photocatalytic adsorbent for wastewater purification.
1. Introduction
Persistent organic pollutants from industry or urban regions in the aquatic ecosystem are serious environmental issues because of their high toxicity, solubility, persistence and carcinogenicity [1,2]. For instance, antibiotics like tetracycline (TC), widely used in human health, animal husbandry and fish farming against infectious diseases, can induce the development of antibiotic-resistant pathogens and cause serious problems for human health and ecosystem balance when they enter into aqueous environments [3,4]. Antibiotic concentrations in raw domestic wastewater are usually reported in the range from 100 ng/L to 6 mg/L [5]. Therefore, an effective and economical technique needs to be urgently developed to remove antibiotic before releasing wastewater into the aquatic environment.
Various technologies have been reported to eliminate antibiotic, such as adsorption, microbial degradation, electrolysis, photocatalysis and membranes separation [6,7]. Among these methods, integrated adsorption and degradation are considered to be one of the most attractive and potential methods for organic pollution and heavy metal ions purification [8]. Semiconductor-based photocatalysis is considered as an effective technology for solving the current environmental problems with utilization of solar energy. The technology could degrade and mineralize organic pollutants into CO2 and H2O under mild condition. Integrated photocatalytic adsorbents such as activated carbon supported TiO2 and hydrogen-titanate nanofibres have high adsorption affinity and photocatalytic activity for organic pollutants removal [9,10]. However, TiO2-based photocatalytic process suffers from some main technical barriers that limit its commercialization, i.e., the inefficient exploitation of visible light (λ > 420 nm), low adsorption capacity for hydrophobic pollutants, uniform distribution in aqueous suspension and difficult post-recovery of the TiO2 particles after water treatment. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants had been reported by our groups [11]. Therefore, in order to enhance the properties of TiO2-based photocatalysts for pollutant purification in real application, exploring novel TiO2-based composites with strong absorbance for broad ranged visible lights, high efficiency and relatively large particles for easily reuse is emergently necessary.
Recently, significant progress about porous materials has been made due to their huge porosities, designable pore structures, and facile modification. Metal–organic frameworks (MOFs), consisting of organic linkers and metal-oxo clusters, have intriguing crystalline structures, tailorable chemistry, large specific surface area and well-defined porosity [12,13]. Consequently, much attention has been paid to potential applications of MOFs in gas capture and storage, chemical separation, sensor devices, drug delivery and catalysis [14-16]. Nevertheless, MOFs were not only served as adsorbents for the removal of acid gases and dye, but also considered as excellent photocatalysts in photocatalysis. For example, Vaesen et al. had demonstrated that the MIL-125(Ti)-NH2 was a promising candidate for the capture of acid H2S and CO2 gases due to the presence of accessible –OH and –NH2 sites together with the absence of Lewis acid sites and high adsorption values [17]. Guo et al. had also reported that pure MIL-125(Ti) could be an adsorbent for the removal of Rhodamine B from aqueous solution [18]. Dan-Hardi et al. had synthesized a photoactive titanium dicarboxylate (MIL-125(Ti) or Ti8O8(OH)4–(O2C–C6H4–CO2)6) and observed a reversible photochromic behavior induced by alcohol adsorption under ultraviolet irradiation [19]. The MIL-125(Ti), a crystalline titanium dicarboxylate with large surface area and accessible pore diameters, has thermal stability and excellent photochemical properties. This material can not only introduce high density of the immobilized Ti sites within porous structure, but also tune the photocatalytic properties via various modified technologies. Nevertheless, pure MIL-125(Ti) photocatalysts is only efficiency in ultraviolet light area and instability during photochemical operations in aqueous solution.
Recently, our groups had previously modified MIL-125(Ti) via using the 2-aminoterephthalicacid as organic link or graphitic carbon nitride as the supporter of MIL-125(Ti) grown [20,21]. It had indicated that these photocatalysts exhibited excellent photocatalytic activity for Cr(VI) reduction and dye mineralization in wastewater under the visible light irradiation. Metal sulfides, a major group of abundant and cheap minerals such as CdS, SnS2, Sb2S3 and so on, have been demonstrated as visible photocatalysts for the removal of pollutants in aqueous solution [22]. Indium(III) sulfide (In2S3) with various morphologies including nanoplates, nanotubes, hollow microspheres and nanorods has attracted great attention due to its good photosensitivity and photoconductivity, stable chemical and physical characteristics and low toxicity [23]. For instance, Wei et al. synthesized flowerlike β-In2S3 microspheres with relatively high visible-light photocatalytic activity for the mineralization of methyl orange [24]. Zhou et al. obtained hierarchical Bi2S3/In2S3 core/shell microspheres with enhanced photocatalytic activity for the degradation of 2, 4-dichlorophenol under visible light irradiation [25]. Interestingly, the hierarchical porous flower-like shell of In2S3 remarkably enhanced the chemical stability of Bi2S3 against oxidation. However, to the best of our knowledge, there has been no report on the preparation and applications of core-shell In2S3@MIL-125(Ti) composite as integrated photocatalytic adsorbent for the removal of antibiotics in wastewater.
The present work quantitatively investigates the adsorption of antibiotics (e.g. tetracycline) on core-shell In2S3@MIL-125(Ti) hybrids. Effects of different ratio of MIL-125(Ti) to In2S3, pH, ion strength and initial concentration on removal of TC were carried out. The adsorption kinetic and isothermal was subsequently studied. Further, the photocatalytic performance, mechanism and reutilization of the composite for the removal of TC were also investigated for potential applications. At last, the IPA for the removal of various real water samples including medical wastewater, municipal wastewater and river water via the integrated adsorption and photocatalytic process were preliminary explored.
2. Experimental
2.1. Materials
Tetrabutyl titanate (TBT; C16H36O4Ti), 1,4-benzenedicarboxylic acid (BDC; C8H6O4), N’N-dimetylformanide (DMF; (CH3)2NCHO), methanol (CH3OH), Indium Nitrate (In(NO3)3·xH2O), Carbon Disulfide (CS2) and Thioacetamide (CH3CSNH2), were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All reagents and solvents were of analytical reagent grade and used as received from commercial suppliers. River water (the value of chemical oxygen demand is 12.6 mg/L) was taken from Xiangjiang River located in Changsha, Hunan province, China. Medical wastewater (the value of chemical oxygen demand is 458.6 mg/L) was obtained from a hospital in Changsha, Hunan province, China. Municipal wastewater (the value of chemical oxygen demand is 185.5 mg/L) was obtained from Yuelu sewage treatment plant in Changsha, Hunan province, China.
2.5. Photocatalytic experiment
Photocatalytic activity of core-shell In2S3@MIL-125(Ti) composites were tested by the photocatalytic decomposition of TC with visible light illumination (λ>420 nm) after adsorption process. A 300 W Xenon lamp (CEL-HXF300, Beijing CEL Tech. Co., Ltd) with a 420 nm cutoff filter was used as the visible light source (14 V, 16 A, 15 cm far away from the photocatalytic reactor). The light intensity of 300 W Xenon lamp was 300 mW cm-2. For decomposition of TC, 30 mg of photocatalyst was dispersed in 100 mL of 46 mg L-1 TC aqueous solution. Prior to irradiation, the suspension was magnetically stirred in the dark for 60 min to get adsorption–desorption equilibrium between photocatalyst and TC. At certain time intervals, samples were collected via filtrating by 0.22 μm PTFE syringe filters. The TC concentration before and after photo-degradation were monitored with a UV-vis spectrophotometer (UV-2250, SHIMADZU Corporation, Japan). The TC concentration after adsorption equilibrium is acted as the initial concentration (C0). Additionally, the recycle experiments were also carried out three consecutive cycles to test the durability and recyclability. After each cycle, the photocatalyst was filtrated and washed for three times to remove residual impurities, and then dried at 60°C for the next test.

3. Results and discussion
3.1 Characteristics of obtained samples
The surface morphology of pure In2S3, pure MIL-125(Ti) and MLS-5 measured by SEM is shown in Fig. 1(a-d). It is found that only irregular microparticles with particle sizes of 400~650 nm forms in the absence of MIL-125(Ti). MIL-125(Ti) has a plate-like form and smooth surface without secondary nanostructures. After solvothermal reaction at 150°C in the presence of sulfur source and indium ions, the surface of MIL-125(Ti) becomes rough and the products still remain the original structures. The higher magnification SEM image (Fig. 1d) indicates a large number of In2S3 nanosheets grown onto the surface of MIL-125(Ti) are interconnected with each other, forming the three-dimensional (3D) nanosheets networks as a stable protective shell. The formation of uniform heterostructures may originate from the special characteristics of the MIL-125(Ti), i.e. the large surface area and porous structure, which facilitate the assembly of In2S3 nanosheets. This unique and ordered core-shell structured microparticle with 3D networks provides an abundant porous surface area for the contact between the materials and pollutants, which is of great significance in accelerating the adsorptive and photocatalytic reaction.
The typical TEM image of an individual MLS-5 microparticle is shown in Fig. 2a. The plate-like MIL-125(Ti) with the diameter of 800 nm is coated by a narrow size distribution of In2S3 nanosheets network automatically, indicating the formation of good quality In2S3@MIL-125(Ti) core-shell microparticle, which is well consistent with the SEM micrographs. From the energy dispersive spectrometer (EDS) mapping of the MLS-5 (Fig. 2e-2f), it can be seen that the blue and yellow dots with bright colour assigned to the In and S element, respectively, is uniformly distributed on the surface of In2S3@MIL-125(Ti) microparticle. For comparison, the C,O and Ti element mapping have also been shown in Fig. 2b-2d. EDS spectrum (Fig. 2g) also displays the presence of In and S element in the core-shell microparticle, and the stoichiometric ratio of indium to sulphur is about 0.67, confirming successful fabrication of In2S3. The HRTEM image (Fig. 2h) of the heterostructures displays a clear lattice fringes with the nearest distance of 0.189 nm coinciding with the value for the (440) plane of cubic In2S3. The presence of the diffraction rings in the selected-area electron diffraction (SAED) pattern (inset of Fig. 2h) indicates that the cubic In2S3 nanosheets are polycrystalline. The crystalline structure of pure MIL-125(Ti), In2S3, and MLS were also examined by XRD, as shown in Fig. 3.
For the MLS with different weight addition of MIL-125(Ti), the XRD patterns are analogous to bare In2S3. The peaks located at 2θ = 27.5°, 33.4°, and 47.9° are distinctly indexed to the (311), (400) and (440) crystal planes of cubic In2S3 phase structure (β-In2S3) (JCPDS 32-0456) [23]. Meanwhile, compared to the XRD patterns of pure In2S3, some new peaks at 16.5° and 19.3° in MLS appear. The values of 2θ correspond to the characteristic peak of MIL-125(Ti), indicating the presence of MIL-125(Ti).
The chemical composition and valence state of various elements in the MLS-5 sample have been carried out by XPS measurement. The survey spectrum in Fig. 4a indicates that elements In, Ti, O, S and C exist in the MLS-5 sample while only the peaks of Ti, O and C appear in pure MIL-125(Ti). Fig. 4b shows the regional spectrum of In 3d with two symmetrical peaks at the binding energy of In 3d5/2 (444.2 eV) and In 3d3/2 (451.7 eV). For the spectrum of S 2p in Fig. 2c, the peaks at 160.7 eV and 161.8 eV are attributed to S 2p3/2 and S 2p1/2 transitions, respectively. The spin–orbit separations of In and S are found to be 7.5 eV and 1.1 eV, indicating that the In and S are present as In3+and S2− in the sample, respectively [25,26,31]. Fig. 4d shows Ti 2p spectra of pure MIL-125(Ti) in comparison with that of the hybrids. The binding energy values of Ti 2p3/2 and Ti 2p1/2 at 459.2 and 464.9 eV, respectively, asserts the existence of Ti4+ for the titanium–oxo cluster [21]. However, a small shift toward lower binding energy is observed for MLS-5 as compared to pure MIL-125(Ti). This may be resulted from the higher ionization energy of sulfur in comparison with that of titanium [32]. The binding energy of 529.7 eV and 531.3 eV of O 1s spectrum (Fig. 4e) may be ascribed to oxygen in titanium–oxo cluster and surface hydroxyl/ether groups. Meanwhile, in comparison with pure MIL-125(Ti), a negative shift of the O 1s with an order of 0.8 eV is also observed for the MLS-5 sample. The shift order of O 1s energy position is the almost same as that of the Ti 2p peak position, implying the incorporation of sulfur in MIL-125(Ti) via S-Ti-O bond. These results are also the proofs for the formation of In2S3@MIL-125(Ti) core-shell heterostructures via chemical synthesis.
The N2 adsorption–desorption isotherms and the corresponding calculated parameters have shown in Fig. 5a and Table 1. The as-obtained samples are of type IV isotherm according to the IUPAC (International Union of Pure and Applied Chemistry) classification. Compared with pure MIL-125(Ti), MLS samples attain a decreased surface area and total pore volume (Vt). The phenomenon can be explained by the fact that a large amount of In2S3 nanosheets, grown on the surface of MIL-125(Ti), block the cavities or pores [21,22]. However, pore size with the diameter of 3.7~3.8 nm shows negligible change after the introduction of In2S3. Combining with the XRD results, it may be inferred that the current approach preserves the structural properties of MIL-125(Ti). The UV-vis absorption spectra were used to determinate the optical property of as-obtained samples. In Fig. 5b, pure In2S3 absorbs the light from the UV to visible-light, and its band gap absorption edge is around 600 nm, while no obvious absorption peak in the visible area is detected for MIL-125(Ti). As for MLS, a notable absorption extension in the visible-light region can be observed due to the In2S3 serving as the visible-light sensitizer. The absorption edges display a gradual blue-shift with increasing the addition amount of MIL-125(Ti) since the band gap of MIL-125(Ti) is wider than that of In2S3. According to the calculation of band gap energy in supporting information, the band gap energy (Eg) of In2S3 is 2.05 eV. As shown in Fig. S1, the band gap for MLS-1 is 2.28 eV, which is lower than that of pure MIL-125(Ti) (3.68 eV). Therefore, the formed heterojunction via the combination of In2S3 and MIL-125(Ti) provide an efficient utilization of visible light, thus producing more electron–hole pair. These results imply MLS are potential photocatalyst for the degradation of pollutants in wastewater under visible light irradiation.
3.2 Effect of various conditions on TC adsorption
3.2.1 Different MLS for TC adsorption
TC adsorption of various MLS samples is investigated and the results are shown in Fig. 6a. Apparently, poor adsorption capacity (14.2 mg/g) has been obtained on the single MIL-125(Ti). An adsorption quantity of 84.9 mg/g is shown for pure In2S3. The MLS system exhibits enhanced adsorption performance and the adsorption capacity of TC increases initially with increasing MIL-125(Ti) mass, and then decreases as the mass further increases. The MLS-5 sample exhibits the highest adsorption performance and the adsorption amount of TC could be reach to 119.2 mg/g after 300 min. The improved performance may be due to that the presence of In2S3 enhances the complex interaction between the exposed indium ions on In2S3 nanosheets surfaces and the polar functional groups of TC molecules [8]. In addition, the introduction of In2S3 nanosheets improves the electronegativity of MLS-5 compared to pure MIL-125(Ti) (as shown in Fig. S2), which is helpful for the removal of positively charged TC molecules. With the increase mass of MIL-125(Ti), the enhanced adsorption is due to the increased hydrogen bonding between the μ-OH groups (of the MIL-125(Ti)) and nitrogen atom of TC, and the π-π interaction between the organic link of MIL-125(Ti) and tetracycline molecules [17,33]. However, MLS-7 shows the lowest adsorption capacity, which may be the smaller specific surface area (Table 1) and the prominent effect of electrostatic repulsion between the positively charged MIL-125(Ti) and the positively charged TC molecules. The mechanism of TC adsorption onto MLS may be the synergistic effect of surface complexation, π-π interactions, hydrogen bonding and electrostatic interactions, as shown in Fig. 6b.