Service hotline
+86 18518316054
 Current location : Home page > Resources > Papers > N-doped TiO2 Nanobelts with Coexposed (001) and (101) Facets and Their Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production
Current location : Home page > Resources > Papers > N-doped TiO2 Nanobelts with Coexposed (001) and (101) Facets and Their Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production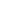
Abstract: In order to narrow the band-gap (3.2 eV) of TiO2 and extend its practical applicability under sunlight, the doping with non-metal elements has been used to tune TiO2 electronic structure. However, the doping also brings new recombination centers among the photoinduced charge carriers, which results in a quantum efficiency loss accordingly. It has been proved that the {101} facets of anatase TiO2 are beneficial to generating and transmitting more reductive electrons to promote the H2-evolution in the photoreduction reaction, and the {001} facets exhibit a higher photoreactivity to accelerate the reaction involved of photogenerated hole. Thus, it was considered by us that using the surface heterojunction composed of both {001} and {101} facets may depress the disadvantage of N doping. Fortunately, we successfully synthesized anatase N-doped TiO2 nanobelts with a surface heterojunction of coexposed (101) and (001) facets. As expected, it realized the charge pairs spatially separation and showed higher photocatalytic activity under a visible-light ray: a hydrogen generation rate of 670 µmol h-1 ·g -1 (much higher than others reported previously in literatures of N-doped TiO2 nanobelts).
1. Introduction
The energy poverty induced by the large number of combustible fossil burning has resulted in an ambitious search for the alternative energy recourses, which are both renewable and environmental-friendly.1 Since the phenomenon of water generating hydrogen on a TiO2 photoanode is found in the 1970s,2 it has drawn numerous attention on account of its promising applications.3-5 However, as a typical wide band-gap semiconductor (3.2 eV), it has a high recombination rate of photoinduced electron and hole pairs, and its primary absorbance in UV spectrum range, also gravely stunts its practical applications under the sunlight.6 Doping with non-metal element have been demonstrated as a feasible means to solve the above two problems.7 However, the doping also brings new recombination centers among the photoinduced charge carriers, which results in the quantum efficiency loss caused by the charge pair recombination accordingly.8
According to the past work, it has been proved that the surface heterojunction structure composed of various facets,9 which have different transmission properties and reactivity for electrons and holes, is conducive to improving the charge pairs separation.10,11 In anatase TiO2 crystal, the {101} facets with higher thermodynamic stability often generate and transmit more reductive electrons in hydrogen evolution from the photoreduction reaction.12,13 Li et al have synthesized N-doped TiO2 nanocatalyst with mainly exposed (101) facets which has a hydrogen production rate of 14.75 µmol h-1 g -1 . 14 Differently, TiO2 nanostructures containing {001} facets exhibited a high photoreactivity to accelerate the photogeneration of holes,15,16 as shown in Figure 1a. Moreover, N-doped TiO2 nanocatalyst with mainly exposed (001) facets has a hydrogen production rate of 211 µmol h-1 g -1 under the visible-light.17 It is seen that N-doped TiO2 with a single exposed facet presents a low H2-production rate. Therefore, the surface heterojunction composed of both {101} and {001} facets has been proposed and it should be more effective to separate the charge pairs (see Figure 1b).18,19 As illustrated in it, the Fermi level of {001} approaches the top of {101} surface in valence band.9 Because of its contact with {101} and {001} facets, the Fermi levels of both the above facets should be equal. Consequently, it is hardly amazing that the surface heterojunction fabricated of both {101} and {001} (as shown in Figure 1b),20 favors both separation and transfer of the carriers. In addition, this structure would also narrow the bandgap of TiO2, favoring the visible light absorbed.
Based on the above comprehensive analysis, herein we prepared a serial of N-doped TiO2 nanobelts with coexposed (001) and (101) facets in surfaces, which are 90 nm wide, 15 nm thick and 7 micrometers long. The unique shape of nanobelts provides the well-defined nanostructures with both (101) facet and (001) facet exposed surfaces. As expected, these N-doped TiO2 nanobelts exhibit a highest hydrogen production rate of 670 µmol h-1 g -1, which is much higher than others reported previously in literatures of conventional TiO2 nanobelts (only several µmol h -1 g -1).16,21
2. Experimental Section
Synthesis of N-doped TiO2 nanobelts: TiO2 nanobelt precursors were synthesized according to the literature.36 0.2g of TiO2 precursor was dispersed into 8 mL deionized water and 12 mL ethanol in a 50 ml glass beaker. After that, an appropriate amount of ammonium hydroxide was added under stirring. After 2 h, the mixture was transferred into a 50 mL Teflon autoclave and maintained at 180°C for 12 h. After that, the sample was cooled to room-temperature, and collected. The as-obtained product was heated up to 500°C for 2 h. The samples of N-doped TiO2 nanobelt were marked as n-N-TiO2.
Characterization: Powder X-ray diffraction (XRD) data was collected by a D/max-rA X-ray diffractometer (Japan Rigaku) with graphite monochromatized Cu-Kα (α = 1.54178 Å). X-ray photoelectron spectroscopy (XPS) measurements were done on an ESCALab220i-XL electron spectrometer and were corrected by a C 1s at 285.0 eV. Scanning electron microscopy (SEM) examinations were conducted on a JEOL-5600LV equipment with a 20 kV accelerating voltage. Transmission electron microscopy (TEM) and the high-resolution transmission electron microscopic (HRTEM) images of N-doped TiO2 nanobelts were obtained by a JEOL HRTEM equipment. The Brunauer-Emmett-Teller (BET) surface areas of the samples were obtained based on a nitrogen adsorption in a Micromeritics ASAP 2020. Diffuse reflectance UV-vis spectra measurements of the samples were proceeded by a UV2550, using BaSO4 as a reflectance standard. The room-temperature photoluminescence (PL) spectra were collected by a spectrophotometer of Jobin Yvon Fluorolog 3-221 using a Xe lamp (450 W, 380 nm) as excitation source. The photocurrent measurements were recorded on a semiconductor characterization system (Keithley 4200 SCS) with a Lakeshore probe station and a xenon lamp (300 W, λ > 400 nm).
Photocatalytic H2-production from water splitting: The photocatalytic water splitting was performed in a 500 mL Pyrex reaction cell. A 300 W xenon arc lamp with a filter (UVCUT400: 400-800 nm) was applied as a visible light source to induce the photocatalytic reaction The photocatalytic H2-evolution from water splitting was measured using an CEL-SPH2N equipment. In a typical photocatalytic experiment, 0.1 g catalyst sample was placed into in 100 mL of a methanol solution (20 ml methanol) in the reaction cell. The 0.5 wt.% Pt was conducted by directly dissolving H2PtCl6 in the aforementioned 100 ml mixed solution as co-catalyst.
3. Results and Discussion
The chemical composition measurements based on XPS analysis of the above as-obtained samples were carried out firstly, as shown in Figure 2a, b and c. As expected, similar peaks due to species containing Ti, O and N in all products are presented in the wide scan XPS spectra. As shown in Figure 2a, two peaks at binding energies of 458.6 and 464.2 eV due to Ti 2P3/2 and 2P1/2, respectively, are found for TiO2 samples. In its O 1s spectrum (Figure 2b inset), an obvious peak at 529.6 eV and a shoulder peak at 531.7 eV are detected, which are due to the lattice oxygen in TiO2 17 and physisorbed water22, respectively. After doping with N, the binding energies of Ti 2P3/2 shift from 458.9eV to 457.8 eV and the O 1s peaks from 530.4 eV to 529.3 eV, respectively, as listed in Table 1. In addition, a DFT calculation has pointed out that the doping of nitrogen resulted in a decrease of the energy cost to the formation of oxygen vacancies.23
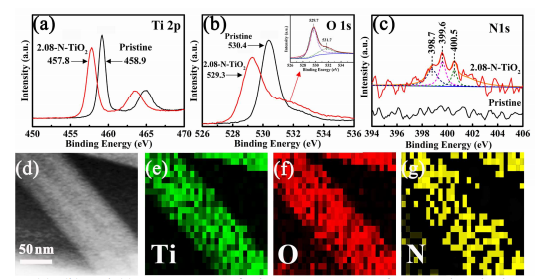
Figure 2. (a), (b) and (c) XPS spectra of Ti 2p, O 1s, S 2p of 2.08-N-doped TiO2 nanobelt, respectively; (d), (e), (f) and (g) TEM image of 2.08-N-doped TiO2 nanobelt and its corresponding EDS element mapping of Ti, O and N elements.
Figure 2c shows the N 1s spectra of 2.08-N-doped TiO2 nanobelts with a broad peak ranging from 398 to 401 eV, which is a characteristic peak of the as-doped nitrogen.24,25 The peak at 398.7 eV (peak 1) is attributed to the replacing O with N (N-Ti-O).26 And the peaks at 399.6 eV (peak 2) and 400.5 eV (peak 3) belong to the Ti-O-N bonding27 and the nitrogen species constrained on surface (essentially adsorbed NOx).28 It would improve the binding energy of the N 1s level. According to the XPS analysis, the contents of nitrogen in TiO2 matrixes obtained by us are 0.53, 0.75, 0.91, 1.32, 1.68 and 2.08 at%, respectively. Beside the XPS results, energy-dispersive X-ray spectroscopy (EDS) mapping is also performed to confirm its element distribution. As shown in Figure 2d, e, f and g, it further confirms the large-scale existence of the Ti, O and N elements, which agrees well with the XPS analysis.
...