Service hotline
+86 18518316054
 Current location : Home page > Resources > Papers > 3D Bi2MoO6 Nanosheet/TiO2 Nanobelt Heterostructure: Enhanced Photocatalytic Activities and Photoelectochemistry Performance
Current location : Home page > Resources > Papers > 3D Bi2MoO6 Nanosheet/TiO2 Nanobelt Heterostructure: Enhanced Photocatalytic Activities and Photoelectochemistry Performance
1.Introduction
In recent years, two-dimensional (2D) flakes decorated on one-dimensional (1D) semiconductor nanostructures to form three-dimensional (3D) heterostructures with versatile properties have been effectively fabricated and highly regarded in various applications, including batteries, photodetectors and photocatalysis1-5. Among these 1D semiconductor nanostructures, TiO2 has attracted great attention in the H2 or O2 production and the decomposition of pollutants6-8. Unfortunately, TiO2 possesses a wide band gap of 3.2 eV and can be photoactivated under UV light, which only accounts for 4% of the solar energy, thus greatly limiting its practical applications9-11 . To further utilize the visible light, which accounts for the main part (48%) of the incoming solar energy12, numerous methods have been made to improve the photocatalytic activity of TiO2-based photocatalysts, such as metallic or non-metallic element-doping13, and hydrogenation or reduction of TiO2 to enhance the light absorption14. In addition, coupling TiO2 with an excellent visible semiconductor photocatalyst to form the heterostructure, such as Ag2O/TiO2, NiO/TiO2, and CeO2/TiO2 15-17, has become an efficient approach for improving the photocatalytic property of TiO2. Because the heterostructure can not only broad the spectral response range to visible light but also promote the charge separation18, 19 .
2.Experimental Section
2.1. Materials. Titania P25 (TiO2), sodium hydroxide (NaOH), hydrochloric acid (HCl), sulfuric acid (H2SO4), ethylene glycol, bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), sodium molybdate dihydrate (Na2MoO4·2H2O) and ethanol were purchased from Sinopharm.
2.2. Preparation of TiO2 nanobelts. P25 (0.2 g) were immersed in 40 mL (10 M) NaOH solution. The suspension was transferred to a 50 mL Teflon-lined autoclave and maintained at 180 oC for 48 h. After washing thoroughly with deionized water, the obtained products were dissolved in (0.1 M) HCl solution for 48 h to obtain H2Ti3O7 nanobelts. Then the above products were immersed in 0.02 M H2SO4 solution and maintained at 180 oC for 10 h. After washing thoroughly with deionized water, the sample was annealed at 600 oC for 2 h.
2.3. Preparation of 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure. Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures (mole ratios from 1:1 to 4:1) were synthesized by a co-precipitation hydrothermal method. Bi(NO3)3·5H2O (0.4-1.6 mmol), Na2MoO4·2H2O (0.2-0.8 mmol) and TiO2 nanobelts (0.2 mmol) were immersed in 15 mL ethylene glycol, respectively, and then were mixed together. The resulted suspension was maintained at 160 oC for 24 h in 50 mL Teflon-lined autoclave. Finally, the products were washed thoroughly with deionized water. For comparison, pure Bi2MoO6 nanosheets were also synthesized by a same fashion without the adding of TiO2 nanobelts.
2.4. Characterizations. X-ray powder diffraction (XRD) measurements of catalysts were conducted on a Bruke D8 Advance X-ray diffractometer using Cu Kα (λ=0.15406 nm) radiation. Scanning electron microscope (SEM) was performed with a HITACHI S-4800 instrument with an energy-dispersive X-ray spectroscopy (EDS). Transmission electron microscope (TEM) were carried out with a JOEL JEM 2100F field emission transmission electron microscope. Fourier transform infrared (FTIR) spectra were recorded on a Thermo Nicolet Avatar 370 FTIR spectrometer in KBr pellets. The UV-Vis diffuse reflectance spectra (DRS) were tested with a spectrophotometer (UV-2550, Shimadzu). The photoluminescence (PL) spectra were measured with a Raman spectroscope (HR 800, JY) under the laser excitation of 325nm.
2.5. Photocatalytic activity test. The photocatalytic activity of the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures was examined towards photodegradation of MO and photocatalytic oxygen evolution. In a photodegradation experiment, 20 mg of the samples were added to 20 mL aqueous MO solutions in an XPA-photochemical reactor (Xujiang Electromech-anical Plant, Nanjing, China). A 350 W mercury lamp was used as the UV light resource. The 300 W Xe arc lamp with filter UV light glasses was used as the visible light source. In a photocatalytic oxygen evolution experiment, catalyst (0.3 g) was added into 100 mL 0.05 mol/L AgNO3 aqueous solution. The reaction temperature was maintained at 5oC. A 300 W Xe arc lamp (CEL-HXF300) was used as the light source. The amount of O2 evolved was determined with a gas chromatograph (Techcomp GC7900).

3. Results and Discussion
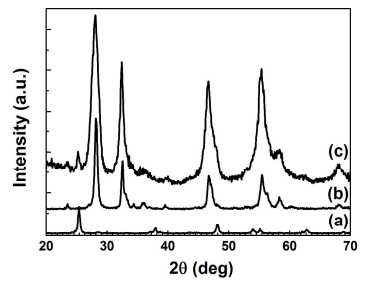
Figure 1. Typical XRD patterns of (a) TiO2 nanobelts, (b) Bi2MoO6 nanosheets, and (c) Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures.
3.1. Structure, Composition, and Morphology. The XRD patterns of TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures are shown in Figure 1. For TiO2 nanobelts (curve a), six distinctive peaks at 2θ=25.28°, 37.80°, 48.05°, 53.89°, 55.06°, and 62.69° match well with anatase TiO2 (JCPDS 21-1272)17. In curve b, the diffraction peaks at 28.25°, 32.59°, 33.07°, 46.72°, 47.07°, 55.46°, 55.53° and 56.16° are observed. These peaks could be perfectly indexed to the (131), (002), (060), (202), (260), (331), (133) and (191) planes of orthorhombic Bi2MoO6 (JCPDS 76-2388)23. For Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures (curve c), all the peaks can be assigned to TiO2 or Bi2MoO6, and no extra peaks are found.
Top inset of Figure 2a presents SEM image of the TiO2 nanobelts, which are 100-300 nm in width, 30-50 nm in thickness, and tens of micrometers in length26 . After acid etching process, surface-coarsened TiO2 nanobelts are obtained (Figure 2a). They exhibit a high specific surface area and provide abundant nucleation sites for the assembling of Bi2MoO6 nanosheets. The Bi2MoO6 nanosheets (Figure 2b) synthesized by the hydrothermal method self-assemble to form Bi2MoO6 microspheres. These spheres are 2-3 µm in diameter. As shown in Figure 2c and d, the surface of TiO2 nanobelts is homogeneously covered with a layer of dense Bi2MoO6 ultrathin nanosheets (mole ratio 2:1). Thus, the 3D-networks are formed, which have a highly porous surface morphology and can act as a fast carrier transfer channel. Moreover, Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures with the different mole ratios (1:1 and 4:1) are also obtained. The corresponding SEM images are presented in Figure S1. Energy-dispersive x-ray spectroscopy (EDS) (Figure S2) shows that Bi, Mo, O elements are found in the Bi2MoO6 nanosheets, and Ti, Bi, Mo, O elements are found in the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures, and no other impurities are observed in the spectra.
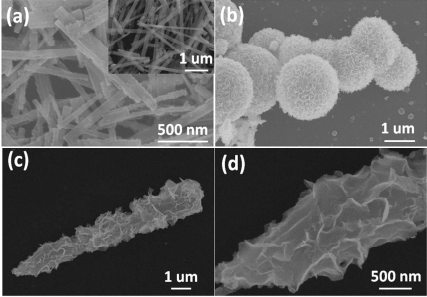
Figure 2. SEM images of (a) surface-coarsened TiO2 nanobelts, TiO2 nanobelts (top inset), (b) Bi2MoO6 nanosheets, and (c, d) Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures.
The TEM image in Figure 3a shows that TiO2 nanobelt has a diameter of about 200 nm, which matches well with the above SEM result (Figure 2a). After assembling Bi2MoO6 nanosheets on the surface of TiO2 nanobelts, the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure still keeps one-dimensional morphology. Bi2MoO6 nanosheets with a very thin layer could be observed on the surface of TiO2 nanobelts, which can be seen in Figure 3b. The high resolution TEM images (HRTEM) of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures show in Figure 3c and d revealing the interplanar spacing of 0.351 nm and 0.315 nm, which correspond to the (101) crystal planes of anatase TiO2, and the (131) crystal planes of orthorhombic Bi2MoO6, respectively, indicating the formation of heterostructures27. The as-fabricated heterostructure have faster charge separation and more efficient carrier transfer compared with pure TiO2 nanobelts or Bi2MoO6 nanosheets, and hence the photocatalytic activities are improved.
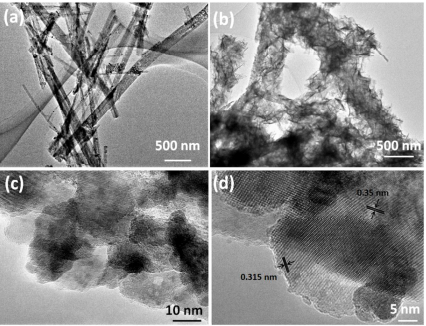
Figure 3. TEM images of (a) TiO2 nanobelts and (b) Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures; (c, d) high-magnification TEM images of Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures.
3.2. Photocatalytic performance. The photocatalytic properties of the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures can be investigated by decomposition of aqueous MO solution under both UV and visible light irradiation (Figure 4). For comparison, Bi2MoO6 nanosheets, TiO2 nanobelts, and P25 were used as photocatalytic references under the same experimental conditions. Before the photocatalysis, blank experiments were measured under below conditions: (1) To achieve the adsorption equilibrium, the solution including MO and photocatalysts was stirred in the dark for 30 min and (2) without photocatalyst under UV and visible light irradiation. Above results illustrated that the samples by themselves exhibited no catalytic activity or absorption on MO in the dark (Figure 4). After 80 min in the absence of photocatalysts, the light irradiation hardly decomposed MO (Figure S3). It can be clearly seen that Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures display enhanced degradation efficiency under UV and visible light irradiation.
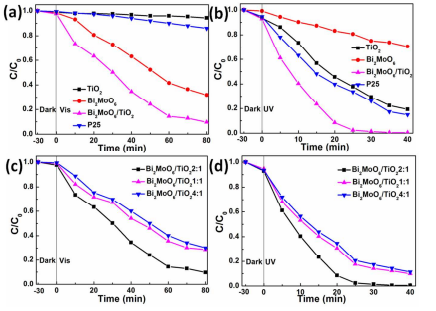
Figure 4. Photocatalytic decomposition of MO for P25, TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures under (a) visible and (b) UV light irradiation; Photocatalytic decomposition of MO over Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures with different mole ratios under (c) visible and (d) UV light irradiation.
We see from Figure 4a that the TiO2 nanobelts and P25 display a low photodegradation rate of MO under visible light irradiation because of their large band gap. The photodegradation rate of Bi2MoO6 nanosheets reaches 68.1% after 80 min visible light irradiation. However, 90.4% of MO is photodegraded by the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures, which is better than that of Bi2MoO6 nanosheets, TiO2 nanobelts (5.7%), and P25 (14%). Under UV light irradiation (Figure 4b), almost 100% of MO is photodegraded by Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures after 40 min, which is much better than that of P25, TiO2 nanobelts, and Bi2MoO6 nanosheets. The degradation rates of MO for the pure TiO2 nanobelts, Bi2MoO6 nanosheets and P25 are 80.7%, 29.6% and 85.1% under 40 min UV light irradiation, respectively (Figure 4b). All these measurements show that the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures exhibit more prominent photocatalytic activity compared to the TiO2 nanobelts or Bi2MoO6 nanosheets alone.
The photocatalytic activities of the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures with different Bi2MoO6/TiO2 mole ratios are also studied (Figure 4c and d). As the increase of Bi2MoO6/TiO2’s mole ratio, the photocatalytic activities of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures increase firstly, achieve a peak value at Bi2MoO6/TiO2=2:1, and then decrease. The probable reasons are put forward as follows: Under visible light irradiation, at the low mole ratio (1:1), the photocatalytic property of TiO2 nanobelt is improved by covering with Bi2MoO6 nanosheet, which is a good visible photocatalyst and has a good light absorption in the visible region24. When the Bi2MoO6/TiO2 mole ratio increases to 2:1, the photocatalytic property of the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures can be enhanced with the increase of Bi2MoO6 amount. After the maximum, the photocatalytic activity of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure decreases with the increase of Bi2MoO6/TiO2 mole ratio (4:1). This should be caused that excessive Bi2MoO6 cover the active sites of TiO2 nanobelts (Figure S1c and d), which hinder the electron transfer on the interface of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures, and thus in turn inhibit the photoactivity. For UV irradiation, when the mole ratio is 1:1, the photocatalytic property of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure is a litter better than that of TiO2 nanobelts. When the mole ratio of Bi2MoO6/TiO2 is 2:1, the uniform layer of Bi2MoO6 nanosheets covered onto the TiO2 nanobelts forming the heterostructures induce the best UV photocatalytic property. The heterostructures can promote the separation of photogenerated carriers, thus the photocatalytic properties are improved. After the mole ratio of Bi2MoO6/TiO2 increases to 4:1, the photocatalytic property drops with the increase of Bi2MoO6/TiO2 mole ratio, because aggregated Bi2MoO6 nanosheets onto the surface of TiO2 nanobelts have smaller specific surface area. (Figure S1c and d)
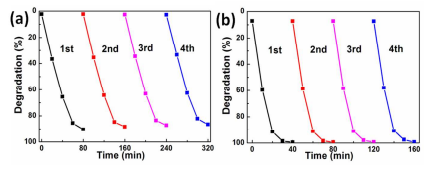
Figure 5. Irradiation-time dependence of photocatalytic decomposition of MO solution for Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures after four cycles under (a) visible and (b) UV light irradiation.
To investigate the photocatalytic stability of the photocatalysts, the photodegradation of MO with the same photocatalysts was measured for four cycles. After each cycle, the photocatalyst was filtered and dried thoroughly, and then the fresh MO solution was added. As is shown in Figure 5, after four cycles, the Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures still keep good photocatalytic stability under UV and visible light irradiation (Figure 5).
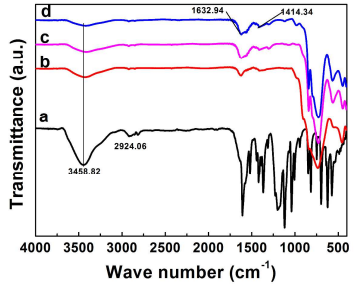
Figure 6. FTIR spectra of (a) methyl orange (MO), (b) Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures, (c) Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures in the MO solution in the dark for 30 min, and (d) Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures in the MO solution under visible light irradiation for 30 min.
The FTIR spectra (Figure 6) were taken on the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures in the MO solution in the dark for 30 min to verify whether MO is absorbed by the heterostructures. The FTIR spectra of MO (Figure 6a) shows peaks at 1036.7 cm-1, 1119.1 cm-1 and 2924.06 cm-1 correspond to ring vibrations, -C-N fingerprints of dye, and -CH3 stretching vibrations, respectively. For the FTIR spectra of pure Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures (Figure 6b), the peak at 1414.34 cm-1, 1632.94 cm-1, 3458.82 cm-1 corresponds to the banding of -OH groups 28, 29. After the heterostructure in MO solutions is kept in the dark and under visible light for 30 min (Figure 6c and d), only the peaks of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures can be observed, and no peaks matching with MO. The total organic carbon (Figure S4) of MO solutions with heterostructure as photocatalyst after visible light irradiation indicates that MO had been well photodegraded.
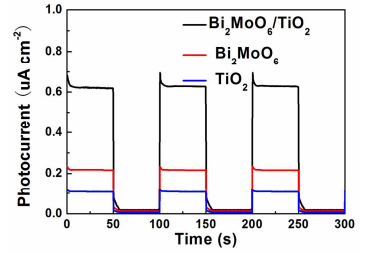
Figure 7. The photocurrent response of the photoanodes with TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures in the dark and under solar simulator irradiation in 0.1 M Na2SO4 solutions.
To further understand the improvement of photocatalytic activity of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures, photoelectochemistry (PEC) performances of TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures as photoanodes were investigated. Figure 7 presents the photocurrent response of samples at a 0.8 V bias vs. SCE under solar simulator irradiation. It can be found that the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures exhibit enhanced photocurrents (70 uA/cm2 ) compared with Bi2MoO6 nanosheets (24 uA/cm2 ) and TiO2 nanobelts (0.9 uA/cm2 ). The higher photocurrent density of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure photoelectrodes indicates an enhanced light absorption and improved separation of photogenerated carriers.
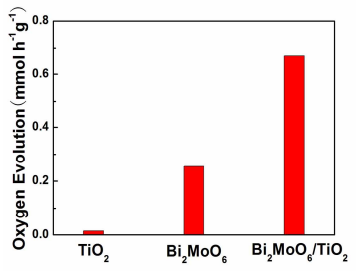
Figure 8. Photocatalytic oxygen production activities of TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures under a Xe arc lamp illumination.
The photocatalytic activity of the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures can also tested by measuring the photocatalytic oxygen production activities (Figure 8). The TiO2 nanobelts present poor photocatalytic oxygen production activity (0.006 mmol h−1g −1), because it can only use the UV light. However, the Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures exhibit the highest oxygen evolution rate of 0.668 mmol h−1g −1, which is better than that of Bi2MoO6 nanosheets (0.254 mmol h−1 g −1) and Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures at other mole ratio (Figure S5). The obvious photocatalytic oxygen production activities can be attributed to the improved visible light absorption of Bi2MoO6 nanosheets24. Importantly, the positive effect from the heterostructure makes the photocatalytic oxygen production activity of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures better than that of Bi2MoO6 nanosheets. Therefore, in the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures, Bi2MoO6 nanosheets are capable visible light harvesters and the heterostructures can provide efficient charge separation, thus the photocatalytic activity of the oxygen production is improved.
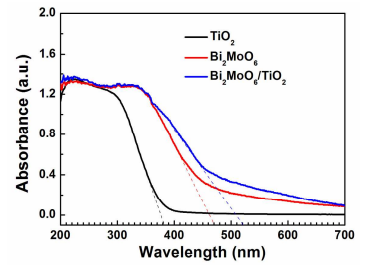
Figure 9. UV-Vis DRS of TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures.
Figure 9 shows UV-Vis DRS of TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures. The absorption of the TiO2 nanobelts is located in the UV region. The absorption cut-off edge is about 393 nm (curve a), which agrees well with the bandgap energy (Eg) of anatase17. However, the absorption edges of the Bi2MoO6 nanosheets extend the absorption edge to 470 nm (curve b). Upon the growth of thin Bi2MoO6 nanosheets on the surface of TiO2 nanobelts, the visible light absorption ability of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures is greatly improved (curve c) in comparison to TiO2 nanobelts, and the absorption edges are located at about 521 nm. Compared with the Bi2MoO6 nanosheets with its absorption edge at 470 nm, the large red shift of absorption of the heterostructure arises from the surface morphological change from sphere to 3D porous structure. This larger absorption would result in the improvement of the photocatalytic property of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures. The absorption edge of TiO2 nanobelts, Bi2MoO6 nanosheets, and Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures is 393 nm, 470 nm, and 521 nm,respectively (Figure 9). Therefore, their Eg is calculated to be 3.15 eV, 2.64 eV, and 2.38 eV, respectively.
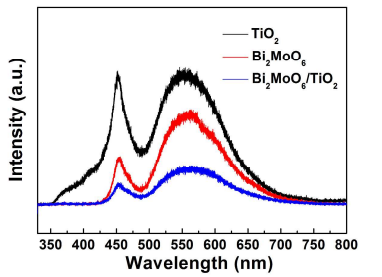
Figure 10. Photoluminescence (PL) spectra of TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures, λEx = 325 nm.
Photoluminescence (PL) spectra can study the separation efficiency of the photogenerated carriers. Figure 10 shows the PL spectra of the TiO2 nanobelts, Bi2MoO6 nanosheets and Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures. The TiO2 nanobelts show two distinct emission peaks at 450 nm and 525 nm, which belong to the emission of the band gap transition17 . The Bi2MoO6 nanosheets also exhibit two strong emission peak, which might due to the intrinsic luminescence properties of Bi2MoO6 30. The Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures show weaker emission peak than that of TiO2 nanobelts and Bi2MoO6 nanosheets, which indicate that the heterostructure between TiO2 and Bi2MoO6 can effectively diminishes the recombination of photogenerated carriers, hence the photocatalytic activity is enhanced.
3.3 Mechanisms of improved photocatatic properties. The improved photocatalytic property of the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures under UV and visible light irradiation may be attributed to the following reasons:
Firstly, according to DRS analysis (Figure 4), Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures have a narrow band gap and exhibit enhanced UV and visible light absorption. The Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures can absorb more UV and visible light than Bi2MoO6 nanosheets and thus the photocatalytic activities are enhanced.
Secondly, the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures have a larger specific surface area. The Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures have higher BET surface area (42.91 m2 g -1) than that of TiO2 nanobelts (19.01 m2 g -1) and Bi2MoO6 nanosheets (26.377 m2 g -1). The high surface area of this 3D heterostructure allows not only more surfaces to be reached by the incident light but also more sites on the surface for the adsorption and photodegradation of MO, which result in enhanced photocatalytic performance. It is also well known that materials with larger specific surface area could adsorb more oxygen on their surface31. The Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures have larger specific surface areas and therefore they could absorb more oxygen (oxygen could react with electrons in the photocatalytic process), which also results in the higher efficiency in the photocatalysis.
In addition, the heterostructure formed between Bi2MoO6 and TiO2 can effectively suppress the recombination of photoelectrons and holes. This is another reason to explain the excellent photocatalytic performance of Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures. In this work, we successfully realize a close contact of Bi2MoO6 nanosheets with TiO2 nanobelts in the Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures. Such close contacts can effectively suppress the recombination between photoelectrons and holes, which results in longer life times for both of them. These well-separated electrons and holes could further take part effectively in the overall photocatalysis. Less of barriers exists between Bi2MoO6 and TiO2 also promote the migration of photogenerated carriers.
At last, 3D porous structure can enhance the photon utilization efficiency and improve the contact between pollutants and photocatalysts. Bi2MoO6 nanosheets covering on the surface of TiO2 nanobelts form the 3D porous structure. A suitable conformation of pores allow a great number of the photons to penetrate deep inside the photocatalyst and many photons remain trapping within the porous structure until being completely absorbed. This might explain why the 3D porous Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures present higher photocatalytic properties than that of Bi2MoO6 nanosheets and TiO2 nanobelts.
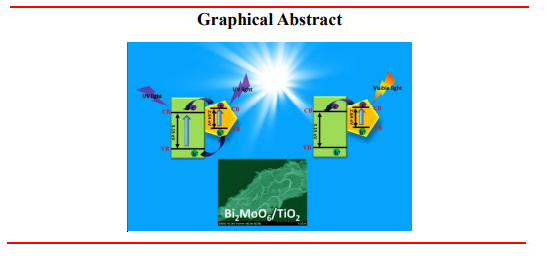
Based on the above discussion, it is obvious that the generation and separation process of electron-hole can be efficiently promoted by the interaction between Bi2MoO6 and TiO2 under UV and visible light irradiation. An electron-hole separation mechanism is shown in Scheme 1. The positions of conduction and valence band can be calculated by the following equation17:ECB=X-Ee-0.5Eg
ECB is the CB edge potential, The X value for TiO2 and Bi2MoO6 is about 5.81 eV and 5.54 eV, respectively17, 32. The Ee value is about 4.5 eV. The Eg value of TiO2 and Bi2MoO6 is 3.15 eV and 2.64 eV, respectively. The ECB values are calculated at about -0.27 eV and -0.28 eV, respectively. Correspondingly, the VB edge potentials (EVB) are estimated to be about 2.88 eV and 2.36 eV, respectively.
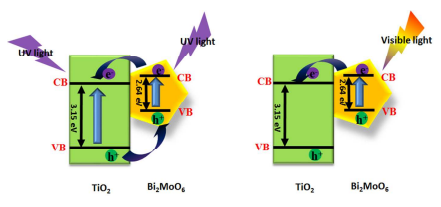
Scheme 1. Schematic diagram of electron-hole separation mechanism upon UV-Vis excitation for Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures.
As shown in Scheme 1, under UV light irradiation, both TiO2 and Bi2MoO6 can be excited, the photogenerated electrons in their CB and holes in the VB generate. Because the EVB of TiO2 (2.88 eV) is more positive than that of Bi2MoO6 (2.36 eV), holes in the VB of TiO2 can migrate to the VB of Bi2MoO6 by the interface. Similarly, the ECB of Bi2MoO6 (-0.28 eV) is lower than that of TiO2 (-0.27 eV), the electrons in the CB of Bi2MoO6 can transfer to the CB of TiO2, thus hindering photoinduced electron-hole recombination in TiO2 and Bi2MoO6, which results in the enhancement of photocatalytic property under UV light irradiation. When exposed to visible light, only the electrons in the VB of Bi2MoO6 can be excited. The electrons in the CB of TiO2 can migrate to the CB of Bi2MoO6, which lead to the separation of carriers.
4. Conclusions
In summary, the novel 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructures are prepared via a simple and efficient hydrothermal method. A dense layer of Bi2MoO6 ultrathin nanosheets are loaded on the surface of TiO2 nanobelts. Importantly, the heterostructure exhibits excellent photocatalytic decomposition of organic dyes properties and water-splitting for oxygen production under UV and visible light. Furthermore, the heterostructure also shows an enhanced photoelectochemistry (PEC) performance and good photocatalytic stability. The enhanced performance can be ascribed to its 3D flake-like porous structure, large specific surface area, large matched energy band of heterostructure, improved charge transfer efficiency and suppressed photoelectron-hole recombination. We believe that the development of the high photocatalytic activities of 3D heterostructures will provide a promising platform for the high-performance photocatalytic applications.
ASSOCIATED CONTENT
Supporting Information. SEM images of Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 1:1) heterostructures and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 4:1) heterostructures. EDS of Bi2MoO6 nanosheet and Bi2MoO6 nanosheet/TiO2 nanobelt (mole ratio 2:1) heterostructures. Photocatalytic degradation of MO for comparison tests. This material is available free of charge via the Internet at http://pubs.acs.org.
AUTHOR INFORMATION
Corresponding Author
* E-mail: hongliu@sdu.edu.cn; cuihongzhi1965@163.com. Phone: +86-531- 88362807. Fax: +86-531- 88362807.
Notes
Any additional relevant notes should be placed here.
ACKNOWLEDGMENT
The authors are thankful for fundings from the National Natural Science Foundation of China (No. 51372142 and 51272141), Taishan Scholars Project of Shandong Province (No. TS20110828), National High Technology Research and Development Program of China (863 Program, No. 2015AA034404), Innovation Research Group (IRG: 51321091) and the “100 Talents Program” of the Chinese Academy of Sciences.
REFERENCES
(1) Wang, L.; Sasaki, T. Chem. Rev. 2014, 114, 9455-9486.
(2) Li, Z.; Wang, F.; Kvit, A.; Wang, X. J. Phys. Chem. C 2015, 119, 4397-4405.
(3) Xiao, F. X.; Hung, S. F.; Miao, J.; Wang, H. Y.; Yang, H.; Liu, B. Small 2015, 11, 554-567.
(4) Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R. I.; Liu, H. Chem. Soc. Rev. 2014, 43, 6920-6937.
(5) Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Chem. Soc. Rev. 2014, 43, 3259-3302.
(6) Chen, J.; Yang, H. B.; Miao, J.; Wang, H. Y.; Liu, B. J. Am. Chem. Soc. 2014, 136, 15310-15318.
(7) Liu, B.; Khare, A.; Aydil, E. S. ACS Appl. Mater. Inter. 2011, 3, 4444-4450.
(8) Zhang, H.; Liu, X.; Li, Y.; Sun, Q.; Wang, Y.; Wood, B. J.; Zhao, H. J. Mater. Chem. 2012, 22, 2465-2472.
(9) Zhou, R.; Zhang, Q.; Uchaker, E.; Lan, J.; Yin, M.; Cao, G. J. Mater. Chem. A 2014, 2, 2517-2525.
(10) Yang, L.; McCue, C.; Zhang, Q.; Uchaker, E.; Mai, Y.; Cao, G. Nanoscale 2015, 7, 3173-3180.
(11) Liu, B.; Liu, L. M.; Lang, X. F.; Wang, H. Y.; Lou, X. W. D.; Aydil, E. S. Energy Environ. Sci. 2014, 7, 2592-2597.
(12) Yang, H. B.; Miao, J.; Hung, S. F.; Huo, F.; Chen, H. M.; Liu, B. ACS Nano, 2014, 8, 10403-10413.
(13) Sakthivel, S.; Kisch, H. Angew. Chem. Int. Ed. 2003, 42, 4908-4911.
(14) Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C. L.; Psaro, R.; Dal Santo, V. J. Am. Chem. Soc. 2012, 134, 7600-7603.
(15) Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. ACS Appl. Mat. Interfaces 2010, 2, 2385-2392.
(16) Lin, J.; Shen, J.; Wang, R.; Cui, J.; Zhou, W.; Hu, P.; Liu, D.; Liu, H.; Wang, J.; Boughton, R. I. J. Mater. Chem. 2011, 21, 5106-5113.
(17) Tian, J.; Sang, Y.; Zhao, Z.; Zhou, W.; Wang, D.; Kang, X.; Liu, H.; Wang, J.; Chen, S.; Cai, H. Small 2013, 9, 3864-3872.
(18) Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. Adv. Mater. 2013, 25, 5075-5080.
(19) Wang, X.; Li, Z.; Shi, J.; Yu, Y. Chem. Rev. 2014, 114, 9346-9384.
(20) Tian, G.; Chen, Y.; Zhai, R.; Zhou, J.; Zhou, W.; Wang, R.; Pan, K.; Tian C.; Fu, H. J. Mater. Chem. 2013, 1, 6961-6968.
(21) Wei, W.; Dai, Y.; Huang, B. J. Phys. Chem. C 2009, 113, 5658-5663.
(22) Zhang, M.; Shao, C.; Mu, J.; Zhang, Z.; Guo, Z.; Zhang, P.; Liu, Y. CrystEngComm, 2012, 14, 605-612.
(23) Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. J. Phys. Chem. B 2006, 110, 17790-17797.
(24) Bi, J.; Wu, L.; Li, J.; Li, Z.; Wang, X.; Fu, X. Acta Mater. 2007, 55, 4699-4705.
(25) Guo, C.; Xu, J.; Wang, S.; Li, L.; Zhang, Y.; Li, X. CrystEngComm 2012, 14, 3602-3608.
(26) Tian, J.; Leng, Y.; Zhao, Z.; Xia, Y.; Sang, Y.; Hao, P.; Liu, H. Nano Energy 2015, 11, 419-427.
(27) Tian, G.; Chen, Y.; Zhai, R.; Zhou, J.; Zhou, W.; Wang, R.; Pan, K.; Tian, C.; Fu, H. J. Mater. Chem. A 2013, 1, 6961-6968.
(28) Fang, Q.; Chen, B. Carbon 2012, 50, 2209-2219.
(29) Tursiloadi, S.; Imai, H.; Hirashima, H. J. Non-Cryst. Solids 2004, 350, 271-276.
(30) Tian, G.; Chen, Y.; Zhou, J.; Tian, C.; Li, R.; Wang, C.; Fu, H. CrystEngComm 2014, 16, 842-849.
(31) Lu, B.; Li, X.; Wang, T.; Xie, E.; Xu, Z. J. Mater. Chem. A 2013, 1, 3900-3906.
(32) Long, M.; Cai, W.; Kisch, H. Chem. Phys. Lett. 2008, 461, 102-105.