Service hotline
+86 18518316054
 Current location : Home page > Resources > Papers > Facile synthesized low-cost MoS2/CdS nanodots-on-nanorods heterostructures for highly efficient pollution degradation under visible-light irradiation
Current location : Home page > Resources > Papers > Facile synthesized low-cost MoS2/CdS nanodots-on-nanorods heterostructures for highly efficient pollution degradation under visible-light irradiation
Facile synthesized low-cost MoS2/CdS nanodots-on-nanorods heterostructures for highly efficient pollution degradation under visible-light irradiation
Leilei Li*, Xingliang Yin*, Yongqiang Sun
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng, 252059, PR China.
*Corresponding author
Abstract:
Semiconductor-based photocatalytic degradation of organic pollutant shows bright prospect and has potential to address the global environmental issues. But the low efficiency, currently, is still an obstacle, which stems from ultrafast charge recombination. In this manuscript, MoS2/CdS nanodots-on-nanorods heterostructures were successfully synthesized via a novel but facile one-pot solvothermal approach with less time and energy consumption. The in-situ synthesized MoS2/CdS heterostructures with intimate interface between MoS2 and CdS facilitate photogenerated charge transportation and separation. Besides, the discrete distribution of MoS2 nanodots on CdS nanorods offers a great many active sites for organic dye degradation. As a result, the optimized MoS2/CdS hybrid shows an excellent photodegradation performance of rhodamine B (RhB) with 99.11% of RhB decomposition in 45 min, which is the advanced among all the similar catalytic systems. Our findings will give inspiration for developing low-cost and efficient catalysts for photodegradation of organic pollutants.
Introduction
Water pollution caused by persistent and nonbiodegradable organic pollutants seriously endangers food safety and human health, and urgently needs to be addressed [1-8]. But it still faces multiple difficulties for thorough water decontamination by conventional approaches, such as high investment and low efficiency. Therefore, it is of great importance to develop low-cost, efficient methods to eliminate organic pollution [9-11]. Photocatalysis as a promising and green technology to decompose organic contaminants has captured particular attention [12-22]. In 1972, Fujishima and Honda first reported TiO2 as an efficient photocatalyst for water splitting [23]. Since then, many semiconductor photocatalysts, such as phosphides, chalcogenides, carbides, oxides and nitrides, have been developed for water splitting, N2 fixation, CO2 reduction and organic contaminant degradation [18, 24-37]. Recently, CdS has spurred considerable interest as a photocatalyst [38-41], due to its low-cost, suitable bandgap (2.4 eV), and favorable conduction band potential. However, the photocatalytic activity of pure CdS still suffers from low efficiency owing to its high-rate charge recombination and serious photocorrosion. In order to improve its photocatalytic performance, various strategies including doping, turning morphology and combing with other semiconductors have been explored [17, 42, 43]. Among them, integrating it with other semiconductors to form heterostructures has proved to be an efficient way to further enhance the photocatalytic performance [13, 32]. The heterostructure not only greatly suppresses charge recombination, but also stabilizes materials [44]. To date, some typical CdS based heterostructures, such as C3N4-CdS [45], CdS/Bi4V2O11 [46], CdS/SrTiO3 [47] and ZnS-CdS [48] have been successfully constructed. Unfortunately, synthesis of those heterostructures requires complicated multi-step process with high time and energy consumption. Therefore, it is still a great challenge to develop a facile approach to fabricate low-cost efficient photocatalysts.
MoS2, a typical low-cost layered transition metal dichalcogenide, has been widely used as a noble-metal-alternative cocatalyst, owing to its peculiar structural and optical properties [49, 50]. MoS2 possesses lower potential of conduction band edge in comparison with CdS [39]. Therefore, combining it with CdS to construct MoS2/CdS heterostructures can accelerate charge transportation and separation, and thus enhance the photocatalytic activity as verified by the previous similar catalytic systems.[51, 52] Very recently, researches indicated that CdS can nucleate at relatively lower temperature compared with MoS2 (160 °C vs. 210 °C) [53-55]. With the help of different nucleation temperatures, MoS2/CdS heterostructures may be constructed via one-pot approach. Inspired by this, we successfully synthesized MoS2/CdS nanodots-on-nanorods heterostructures via a simple one-pot solvothermal method. The obtained heterostructures, where the MoS2 nanodots are tightly anchored on the CdS nanorods, are beneficial for the charge transportation and separation. In addition, this nanostructure and discrete distribution of MoS2 nanodots along CdS nanorods not only shorten the transfer path of electrons and holes, but also preserve plenty of accessible surface for efficient holes extraction by organic dye. As a result, the MoS2/CdS nanodots-on-nanorods heterostructures exhibite extraordinary photocatalytic activities toward RhB degradation under visible light irradiation. Combining the benefits from the facile one-pot synthesis process with less time and energy consumption, the present results may inspire the exploration of low-cost efficient photocatalysts for the degradation of organic pollutants.
Experimental Section
1.1 Photocatalyst preparation
Synthesis of MoS2/CdS: 2 mmol Cd(NO3)2·4H2O and a varying amount of Na2MoO4·2H2O and CS(NH2)2 (The molar ratio of (Cd(NO3)2·4H2O+Na2MoO4·2H2O)/CS(NH2)2 is 1:5) were added into a Teflon-lined, stainless steel autoclave filled with ethylenediamine to 80% of its capacity (100 mL). The autoclave was sealed and heated in an oven at 220 °C for 24 h. After that, the precipitates were collected by centrifugation, and rinsed with water and ethanol. The end products were obtained after vacuum drying at 60 °C for 6 h. Synthesis of pure CdS and MoS2: As a control, pure CdS and MoS2 were also prepared using the same method in the absence of Na2MoO4·2H2O and Cd(NO3)2·4H2O, respectively.
1.2 Characterization
The as-prepared catalysts were characterized by X-ray powder diffraction (XRD, Rigaku D/max-7000) with Cu Kα irradiation (λ = 1.54178 Å). The morphology of the as-prepared catalysts were analyzed on scanning electron microscopy (SEM, JSM-6701F) and transmission electron microscopy (TEM, JEM 2100F). The UV-visible absorption spectra were recorded on a UV-visible spectrophotometer (PerkinElmer Lambda 750). The electronic states of the elements of the obtained products were analyzed using X-ray photoelectron spectroscopy (XPS, VG ESCALab220i-XL) with 300 W Mg Kα irradiation. The binding energies were referenced to the C1s line at 284.8 eV from adventitious carbon. Inductively coupled plasma optical emission spectrometry (ICP-AES, ICPE-9000 Shimadzu) was used to measure the Mo and Cd contents. Brunauer-Emmett-Teller (BET) measurements were performed on a Micromeritics’s Tristar 3000. Transient photocurrent intensity of the samples were measured on an electrochemical workstation (CHI 760 E electrochemical system) using Ag-AgCl as reference and Pt as counter electrode. The work electrode was prepared by dispensing sample suspension in ethanol onto ITO/glass of fixed area (1.96х10-5 m 2 ). The electrolyte is lactic acid solution (1.33 M) which was filled in a quartz cell with a side window for external light incidence. Light on and off was controlled by a baffles installed on a stainless steel black box.
1.3 Photocatalytic performance measurement
The photocatalytic activities of the obtained products were evaluated by degrading RhB in an aqueous solution (10 mg L−1 ) under visible light irradiation. Before photocatalytic reaction, 100 mg photocatalysts were ultrasonically dispersed into a Pyrex photocatalytic reactor containing 100 mL of RhB solution, with cooling water circulating in dividing wall to keep the reaction at 20 °C. The light was introduced from the upside circular quartz window (the reaction system (Beijing China Education Au-light Co., Ltd) see Fig. S1). Then, the mixtures were stirred in darkness for 45 min to ensure the adsorption-desorption equilibrium. After that, the photochemical reactor was illuminated by using 300 W Xe lamp with a 420 nm cutoff filter while stirring. At intervals of 15 min irradiation, 5 mL reaction solution was taken out from the suspension, and centrifuged at 8000 rpm for 4 min to remove the photocatalysts. Finally, the concentration of RhB was measured by a UV-vis spectrophotometer. The degree of degradation was described by C/C0, which is the ratio of the temporal dye concentration to the initial dye concentration.
2. Results and Discussion
2.1 Characterization of as-synthesized samples
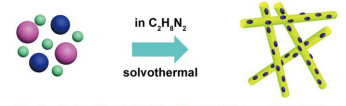
Fig. 1. Schematic illustration of the synthesis process for MoS2/CdS nanodots-on-nanorods hybrids.
The synthesis process of MoS2/CdS heterostructures is schematically illustrated in Fig. 1. All the starting materials (Cd(NO3)2, Na2MoO4, CS(NH2)2) were mixed together and added into the reactor. After solvothermal reaction at 220 °C in ethylenediamine solution for 24 h, the end-products were received. As a control, the pristine CdS was also prepared (for more detailed information about the synthesis process please see the experimental section.) The morphology of the products was investigated by scanning electron microscopy (SEM) and transition electron microscopy (TEM), respectively, and the results are shown in Fig. 2. SEM (Fig. 2a) and TEM (Fig. 2b)
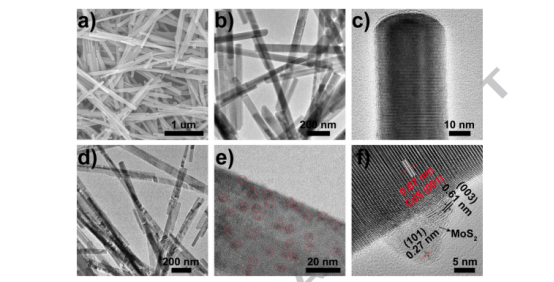
Fig. 2. (a) SEM, (b) TEM, and (c) HRTEM images of pure CdS. (d)TEM, (e) HRTEM, and (f) high-magnification HRTEM images of MoS2/CdS-5 wt.%. images reveal the pristine CdS is composed of typical smooth nanorods with the diameter of 60~80 nm and the length of several micrometers. Besides, no different contrast was observed from its high resolution TEM (HRTEM, Fig. 2c) image. For the optimized MoS2/CdS heterostructure denoted as MoS2/CdS-5 wt.% (5 wt.% : MoS2/CdS), its TEM image (Fig. 2d) is similar to that of pure CdS, but its HRTEM image (Fig. 2e) shows that a great many of low contrast nanodots (marked by red circles as observed in Fig. 2e) in a diameter of several nanometers were intimately loaded on the surface of CdS nanorods. Careful study shows that these nanodots are amorphous structure with discontinuous short-range order lattice fringe (Fig. 2f). The lattice spacing of 0.61 and 0.27 nm for nanodots are well corresponding to the (003) and (101) planes of rhombohedral MoS2. The lattice fringe for the nanorods is continuous long-range order, indicating its good crystallization with single crystal-like
Fig. 3. XRD patterns of MoS2, CdS, and MoS2/CdS heterostructures with MoS2 mass ratio of 5 wt.%, 10 wt.%, and 15 wt.%, respectively, in versus of the JCPDS pattern for standard CdS. structure. The lattice spacing of 0.36 nm can be ascribed to the (100) plane of hexagonal CdS. This intimate contact between MoS2 and CdS makes for the well-defined heterostructure formation, and thus accelerates the photogenerated charge separation
The phase structures of MoS2/CdS nanohybrids as well as pure CdS and MoS2 were further studied by X-ray powder diffraction (Fig. 3). The diffraction patterns of pristine CdS are well-indexed to the hexagonal CdS (JCPDS No. 65-3414). Whereas, the widened peaks of pure MoS2 with nanosheet structure (see Fig. S2) exhibits the low crystallity of MoS2, which is in agreement with that observed from the mentioned-above TEM image of MoS2 in MoS2/CdS nanohybrids. No characteristic patterns of MoS2 were observed even its mass ratio increases to 15 wt.%. Compared with the pristine CdS, there is no apparent shift in the diffraction peak positions for the hybrid samples, manifesting the MoS2 existed in them is in form of loading but not doping. No XRD patterns of MoS2 appeared, here, can be resulted from the low crystallity of MoS2 anchored on the CdS nanorods.
The surface chemical states of the obtained hybrid sample MoS2/CdS-5 wt.% were examined by X-ray photoelectron spectroscopy (XPS). The wide-survey XPS spectrum as shown in Fig. 4a reveals the existence of Cd, Mo, and S. The high resolution spectrum of Cd 3d (Fig. 4b) displays two peaks at 412.0 and 405.1 eV, which are assigned to the characteristic binding energies of Cd2+ 3d3/2 and Cd2+ 3d5/2 in CdS, respectively. Analysis of Mo 3d signal in Fig. 4c, indicates two strong doublet peaks arising from Mo 3d5/2 and Mo 3d3/2, located at 231.4 and 228.2 eV, respectively, suggesting the dominance of Mo4+ in the hybrid sample. The corresponding high-resolution S 2p (Fig. 4d) can be well deconvoluted into two peaks centered at
162.4 and 161.1 eV, corresponding to S 2p1/2 and S 2p3/2 in the form of S2- , respectively. The XPS analysis together with the characterization results of SEM, TEM, and XRD corroborate the successful synthesis of MoS2/CdS nanodots-on-nanorods.
3.2 Photocatalytic properties
Photocatalytic performance of MoS2/CdS nanohybrids with different MoS2 mass ratio, as well as contrast samples of pristine CdS and MoS2 were evaluated by RhB Table 1. Theoretical and actual content of MoS2 in all nanohybrid samples
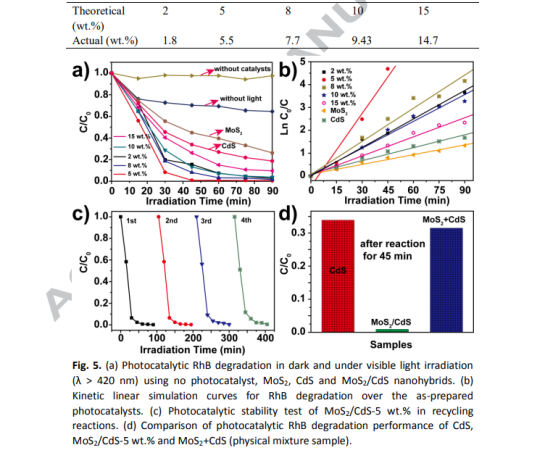
photodegradation under visible light irradiation. All mass ratios mentioned in this manuscript are referred to the theoretical loading capacity of MoS2 versus CdS. The real mass ratios of nanohybrid samples were determined by the inductively coupled plasma atomic emission spectroscopy (ICP-AES), as listed in Table 1. To get maximum yields, the CS(NH2)2 among starting materials is excessive. It can be seen from Table 1 that the real mass ratios are close to the theoretical ones. The temporal evolution of the spectral changes during the photodegradation of RhB at different conditions were presented in Fig. S3. It was noteworthy that original RhB showed a major absorption band centered at around 550 nm being similar with that of other previous reports.[56, 57] The corresponding calculated C/Co values are plotted in Fig 5a. As can be seen, no obvious RhB degradation was observed under visible light illumination in the absence of photocatalysts, which excludes the possibility of a self-photolysis of RhB, and demonstrates its high stability. And only a little amount of RhB (9.35%) could be degraded by MoS2/CdS-5 wt.% without light, which illustrates that visible light plays an important role in the RhB degradation process. Pure MoS2 displays relatively low photocatalytic performance with only 73.55% of RhB degradation for 90 min irradiation. In comparison to MoS2, pristine CdS shows higher photocatalytic activity toward RhB degradation (a removal of 81.23% after 90 min reaction), which is far better than that of other reported ones [45, 58]. The superior performance of CdS nanorods can be ascribed to their nano-morphology and single-crystalline nature, which shorten photogenerated charge migration path from inert to surface, and significantly inhibit bulk charge recombination owing to low bulk
defects in materials. Interestingly, the optimized MoS2/CdS nanodots-on-nanorods (MoS2/CdS-5 wt.%) can further enhance degradation rate with RhB nearly completely degraded (99.11%) in 45 min. To get the photodegradation rate, pseudo-first-order kinetics equation was employed, as follows:
...