Service hotline
+86 18518316054
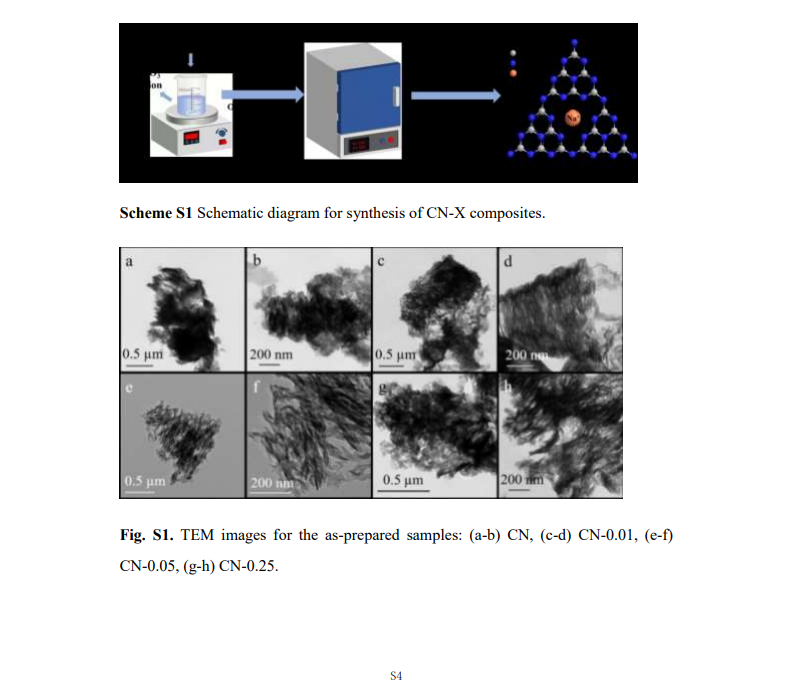
 Current location : Home page > Resources > Papers > One-pot synthesis of sodium-doped willow-shaped graphitic carbon nitride for improved photocatalytic activity under visible-light irradiation
Current location : Home page > Resources > Papers > One-pot synthesis of sodium-doped willow-shaped graphitic carbon nitride for improved photocatalytic activity under visible-light irradiation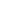
Qian Doua , Jianhua Houa,b,c∗ , Asif Hussaina , Geshan Zhangd , Yongcai Zhange , Min Luof , Xiaozhi Wang a,c, Chuanbao Cao
Experimental section
Characterizations
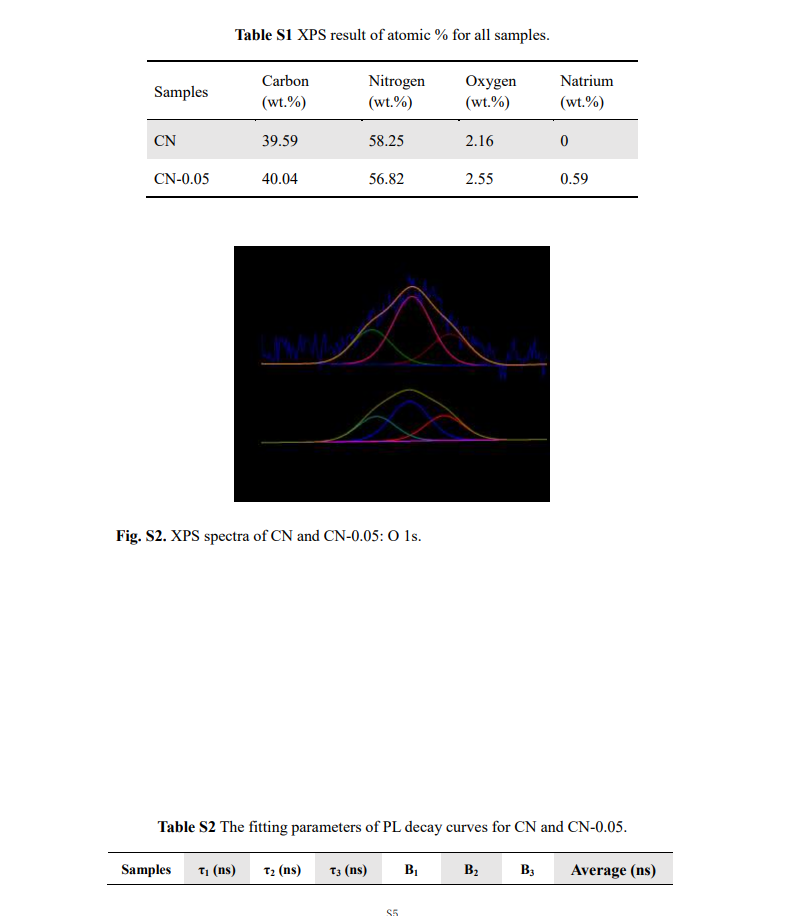
The prepared photocatalysts were analyzed and characterized by using different experimental techniques, such as XRD, FT-IR, XPS, EPR, HRTEM, SEM, DRS, UV-vis, BET, PL and EIS analysis. The XRD (X-ray diffraction) was characterized on AXS D8 ADVANCE, Bruker with Cu Kα radiation using (λ=1.5406 Å). The FT-IR (Fourier Transform Infrared Spectroscopy) measurements were analyzed by TENSOR27 spectrometer. For surface elemental state measurements, the XPS (X-ray photoelectrons spectroscopy) (ESCALAB 250Xi, Thermo Fisher Scientific) was exercised. The EPR (Electron Paramagnetic Resonance) was measured with A300-10/12 Bruker EPR spectrometer. The morphology and microstructure were recognized using HRTEM (High-Resolution Transmission Electron Microscopy) with (Tecnai G2 F30 S-Twin, FEI). The optical property was recorded on the UV-visible diffuse reflectance spectroscopy (UV-Vis DRS) (TU-1901, PERSEE). The specific surface area of composites was measured with The Brunauer-Emmett-Teller (BET) (ASAP 2460, Micromeritics). Photoluminescence (PL) was studied by Renishaw in Via. Electrochemical impedance spectra (EIS) and Mott-Schottky plots were tested by CHI760E electrochemical workstation using the standard three-electrode system, here Pt was used as a counter electrode, Ag/AgCl as the reference electrode, and ITO conductive glass containing 1 mg catalyst sample as the working electrode in 0.2 M Na2SO4 solution. The 0.01-105 Hz frequency range and 5 mV AC voltage were taken into account for EIS measurements. The frequency of the Mott-Schottky test was fixed at 10 and 100 Hz. The concentration of simulated pollutants was measured using an ultraviolet-visible spectrophotometer (UV-2450, SHIMADZU, Japan).
Photocatalytic activity measurements
The Xenon lamp with (power P=500 W, and wavelength λ>420 nm) was used as a visible light simulator. The Rhodamine B (RhB, 10 mg L-1 ) and Methyl Orange (MO, 10 mg L-1 ) organic dyes were exercised as simulated contaminants. 20 mg of the synthetic material was mixed with 50 ml of the contaminants aqueous solution and stirred in the dark for 2 h to reach a catalytic adsorption-desorption equilibrium with the contaminants. Lastly, samples were then taken every 30 min time period in the light. The above degradation experiment was carried out three times in each group. The average result of the three repeated experiments is used as the final result, and the error estimate is represented by the error bar in the figure.
Active species experiments
The BQ (1 mmol L-1 ) or BQ (1 mmol L-1 ) and EDTA-2Na (1 mmol L-1 ) were included in the reaction solution for photogenerated charge carriers such as h+ , hydroxyl radicals (•OH) and peroxyl radicals (•O2 - ), respectively. The additional measures are similar to photocatalytic measurements. The 3 mg samples were mixed in 50 mmol L-1 DMPO - •OH or DMPO methanol or DMPO-•O2 - , respectively
Photocatalytic activity evaluation
The hydrogen generation trials were carried out at 279k in a heat-resistant glass-top irradiated reaction vessel associated with a glass-tight gas system. Every experiment included 20 mg of photocatalyst material, which was distributed in an aqueous solution of about (50 mL) comprising Pt (3 wt%) as a co-catalyst and TEOA (triethanolamine 10 vol%), which was used as a sacrificial electron donor. A Xenon lamp of 300 W (CEL-HXF300/CEL-HXUV300) was exercised as a visible light source and to completely remove air the reaction vessel was evacuated for 30 minutes. The H2 precipitation was analyzed by a gas chromatography system (Beijing China Education Au-light Co., Ltd), which was connected to TCD (thermal conductivity detector) and with a molecular 5A column.
The Pyrex cell reactor enclosed in a closed circular system was used to perform photocatalytic CO2 reduction experiments. The Xenon lamp with a power of 300 watts was taken into account as a visible light source and 20 mg photocatalysts material was dispersed in a glass reactor uniformly. The reaction system is vacuumed various times and CO2 gas (99.999%) purity with pressure (0.08 MPa) has flowed into the reaction system. Subsequently, 2 ml of deionized water as an electron donor was vaccinated into the reaction system. To guarantee that the adsorption of gas molecules proved sufficient, individual photocatalysts were allowed to settle in the CO2/H2O environment for many hours. An automated gas chromatograph was used to examine the gas products (Beijing China Education Au-light Co., Ltd, FID2 detector, argon as carrier gas).

The above Production of hydrogen and Carbon dioxide reduction experiment was carried out three times in each group. The average result of the three repeated experiments is used as the final result, and the error estimate is represented by the error bar in the figure.
The AQY measurement
The apparent quantum yield (AQY) for H2 evolution was measured using monochromatic Xenon lamps with band pass filter of 420 nm. The irradiation area was controlled as 7.54 cm2 . The intensities were 44.71 mW cm-2 respectively (ILT 950 spectroradiometer). The AQY was calculated as AQY=Ne/Np *100% = 2M NA hc/SPt λ*100%, where Ne is the amount of reaction electrons, Np is the amount of incident photons, M is the amount of H2 molecules, NA is Avogadro constant, h is the Planck constant, c is the speed of light, S is the irradiation area, P is the intensity of the irradiation, t is the photo-irradiation time, and λ is the wavelength of the monochromatic light.