Service hotline
+86 18518316054
 Current location : Home page > Resources > Papers > A2V2O7 (A = Co, Ni, Cu and Zn) for CO2 reduction under visible-light irradiation: effects of A site replacement
Current location : Home page > Resources > Papers > A2V2O7 (A = Co, Ni, Cu and Zn) for CO2 reduction under visible-light irradiation: effects of A site replacement
Abstract
Electronic structure and surface character are two important factors of materials for photocatalytic CO2 reduction. Herein, the simultaneous regulation of electronic structure and surface basicity of pyrovanadate A2V2O7 (A = Co, Ni, Cu, Zn) were realized by changing the A-site element. The density functional theory (DFT) results suggested that Ni2V2O7 had the strongest crystal field and the greatest splitting of V 3d, resulting in the largest mobility of photogenerated electrons. Moreover, the Tanabe hypothesis predicted that the A-site cation was a basic surface site, and the CO2-TPD results revealed that Ni2+ has the strongest surface basicity. Ni2V2O7 exhibited much higher CO yield of 33.1 μmol/g, compared to Co2V2O7 (16.7 μmol/g) and Zn2V2O7 (12.9 μmol/g). Cu2V2O7 was not suitable for CO2 reduction due to its positive conduction band position caused by splitting the Cu 3d orbital. Altogether, this study contributes to deeply understanding metal vanadates for photocatalytic CO2 reduction.
Keywords: vanadate; photocatalytic CO2 reduction; electronic structure; basicity
1. Introduction In recent years, reducing CO2 to fuel or other useful carbon compounds under illumination has become a research hotspot.[1-5] It is also considered a potential way to alleviate environmental problems and energy crises. Using this method, solar energy, the most abundant clean energy on Earth, can be efficiently stored as chemical energy in solar fuels and then easily reused. [6, 7] When these solar fuels release energy, the process of “carbon footprint” is zero in principle. To achieve solar-to-fuel conversion efficiency on a practical level, a more efficient harvest of photons in the visible region is indispensable, since visible light accounts for approximately 43% of the total solar energy, while UV light occupies approximately 4%. However, most traditional oxide photocatalysts, such as TiO2 and ZnO, have a relatively wide bandgap (~3 eV) due to the deep O 2p orbitals. [8] Therefore, it is desirable to understand the effects of crystal and electronic structures of photocatalysts on their photocatalytic CO2 reduction. [9] For example, the bandgap and band edge positions in perovskite oxide photocatalysts can be adjusted by changing the A-site or/and B-site cations. [10] Teramura et al. reported ATaO3 (A= Li, Na, K) for photocatalytic CO2 conversion. [11] The photocatalytic activity decreased in the order LiTaO3 > NaTaO3 > KTaO3, corresponding to band gap values of 4.9 eV, 4.1 eV and 3.7 eV for LiTaO3, NaTaO3 and KTaO3, respectively. Ouyang et al. studied a series of Ag-based oxides such as AgAlO2, AgCrO2, and Ag2CrO4. [12] Ag2CrO4 exhibits a much higher photocatalytic activity than AgAlO2 because of a larger Ag–O bond length and O–Ag–O bond angle. These results will help us understand how to choose an applicable semiconductor photocatalyst for CO2 reduction on the basis of thermodynamics.
In addition, surface characteristics, such as acid-basic sites, were closely related to photocatalytic activity, which could affect the adsorption of CO2 in terms of kinetics. Especially for photocatalytic CO2 reduction, the catalyst surface performed basicity was easier to adsorb acidic CO2 molecules. [13] In a class of layered double hydroxides (LDHs), the species and proportion of metal cations in the layers usually affect their surface basicity.[14] Hong et al. studied the CO2 methanation performance of several LDHs and C3N4 composite materials. [15] Among them, the assembled MgAl-LDH/C3N4 had the best catalytic activity and a strong CO2 adsorption capacity, which was attributed to the fact that Mg2+ cations could produce stronger basicity than Zn2+, Cr3+, Al3+, and Ni2+. It is inferred that the species of metal cations in other compounds might also affect surface basicity, and controlling the surface basic sites is a promising strategy to enhance the catalytic performance of CO2 reduction.
Transition meatal vanadate has enormous potential in the application of photocatalytic CO2 reduction because it has a narrow optical band gap (2.0 eV ~ 2.4 eV) due to the existence of the V 3d orbital. [16] Meanwhile, the multiple valences of V from +2 to +5 resulted in variable stoichiometric ratios and diverse crystal structures with transition metal elements, and thus, different electronic structures and photocatalytic activities could be regulated. [17] In this paper, a series of metal vanadates, A2V2O7 (A = Co, Ni, Cu, Zn), were synthesized by a solid-state reaction to probe the phase structure-reactivity relationship. Based on the physicochemical properties and electronic structures, the influences of crystal structures and electronic structures on the photocatalytic activities of A2V2O7 were systematically investigated. In addition, the influence of different metal cations on the surface basicity of the catalyst was also studied, which would affect the CO2 adsorption capacity. These results contribute to deeply understanding and feasibly constructing metal vanadate-containing photocatalysts with high photocatalytic activity for CO2 reduction.
2. Experiment
2.1 Catalyst preparation
The pyrovanadate A2V2O7 were prepared by the conversional solid-sate method. A2V2O7 (A = Co, Ni, Cu, Zn) was prepared by corresponding metal nitrates and equimolar ammonium metavanadate, which were mixed and ground in an agate mortar completely. Mixture was preheated at 350 °C for 2 h in the air while grinding thoroughly. Subsequently, the A2V2O7 precursors were annealed for 2 h at 800 °C, 700 °C, 500 °C and 600 °C, respectively.
2.2 Characterizations
XRD patterns were recorded on a Rigaku Ultimate III diffractometer using Cu Kα radiation (40 KV, 40 mA) to analyze the structure and composition of the samples. SEM images were collected on a Carl Zeiss SMT AG scanning electron microscope. Samples for SEM imaging were deposited on carbon tape. The UV–vis diffuse reflectance spectra were recorded with a UV–vis spectrophotometer (UV-2550, Shimadzu, Japan) in the range of 250-2500 nm at room temperature and transformed into absorption spectra according to the Kubelka-Munk relationship. The X-ray photoelectron spectroscopy (XPS) data were collected using a PHI5000 Versa Probe X-ray photoelectron spectrometer (ULVAC-PHI, Japan). The binding energy was determined by reference to the C 1s line at 284.6 eV. The specific surface area and CO2 adsorption capacities of the samples were determined by BET measurements (TriStar-3000, Micromeritics, USA). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were obtained by a transmission electron microscope (JEM-2100F for Co2V2O7 and Ni2V2O7; Talos F200 for Cu2V2O7 and Zn2V2O7) operated at 200 kV. Mott-Schottky and photochemical experiments were conducted in an aqueous phosphoric acid buffer solution (pH = 6.8) using a CHI760B electrochemical work station. FTO glass with 1×2 cm2 samples deposited was used as the working electrode. A platinum plate and Ag/AgCl electrode (SCE) were used as the counter and reference electrodes, respectively.
2.3 Evaluation of photocatalytic activity
The gas phase photocatalytic reduction was performed in a sealed glass reactor with a volume of 230 mL. The light source was a 300 W Xenon arc lamp (CEL-PF300-T8E, Beijing Au-light, China) equipped with a cutoff filter (λ ≥ 420 nm).
Thirty milligrams of photocatalyst were scattered evenly on glass microfiber filter paper (Whatman, Grade QMA) with an effective area of 2 cm2 by filtering the sample suspension. The reactor containing the sample was first vacuumed and flushed with CO2 several times. After that, high purity CO2 was gradually pumped into the reactor until atmospheric pressure was reached, and 0.4 mL deionized water was injected into the reaction chamber as reactants. Before irradiation, the photocatalyst was kept in the dark for 8 h to achieve adsorption-desorption equilibrium. In the process of the reaction, 1 mL gas was extracted from the chamber at regular intervals with a sampling needle for subsequent concentration analysis. The carbon-based products were analyzed by gas chromatography (GC-2014, Shimadzu Corp., Japan) with a flame ionization detector (FID). The amount of H2 was determined by gas chromatography with a thermal conductivity detector (TCD) (GC9790 Plus, Zhejiang Fuli Analytical Instrument, China).
2.4 Computational Details
All our calculations in this study are performed using the Vienna Ab initio Simulation Package (VASP), which employs the spin-polarized DFT method. The generalized gradient approximation (GGA) in the Perdew-Bueke-Ernzerhof (PBE) scheme is used for the exchange correlation functional. The strong on-site Coulomb repulsion between localized 3d electrons was modified by considering the Hubbard U correction proposed by Dudarev et al., and effective U values of 3.25 eV, 3.32 eV, and 6.2 eV were used for the V 3d,[18] Co 3d, and Ni 3d orbitals, respectively. [19, 20] The cutoff energy is 520 eV, which is high enough to ensure that no Pulay stresses occur within the cell during geometric relaxations in all materials. A 4×3×3, 3×3×3, 4×3×3, 3×3×3 k-point sampling in reciprocal space was used for Co2V2O7, Cu2V2O7, Ni2V2O7, Zn2V2O7, respectively. Geometry relaxations are carried out until the residual forces on each ion converge to be smaller than 2×10–5 eV/Å.
3. Results and Discussion
3.1 Phase and crystal structure of A2V2O7 (A = Co, Ni, Cu, Zn)
The target compounds A2V2O7 (A = Co, Ni, Cu, Zn) were synthesized by a solid-state method. The powder X-ray diffraction patterns of Co2V2O7, Ni2V2O7, Cu2V2O7, Zn2V2O7 are shown in Figure 1. These photocatalysts are crystallized in a single phase. Figure 2 lists the lattice parameters of these bimetallic vanadium oxides. It was found that Co2V2O7 and Ni2V2O7 are monoclinic, space group P21/c, while Cu2V2O7 and Zn2V2O7 are monoclinic, space group C2/c
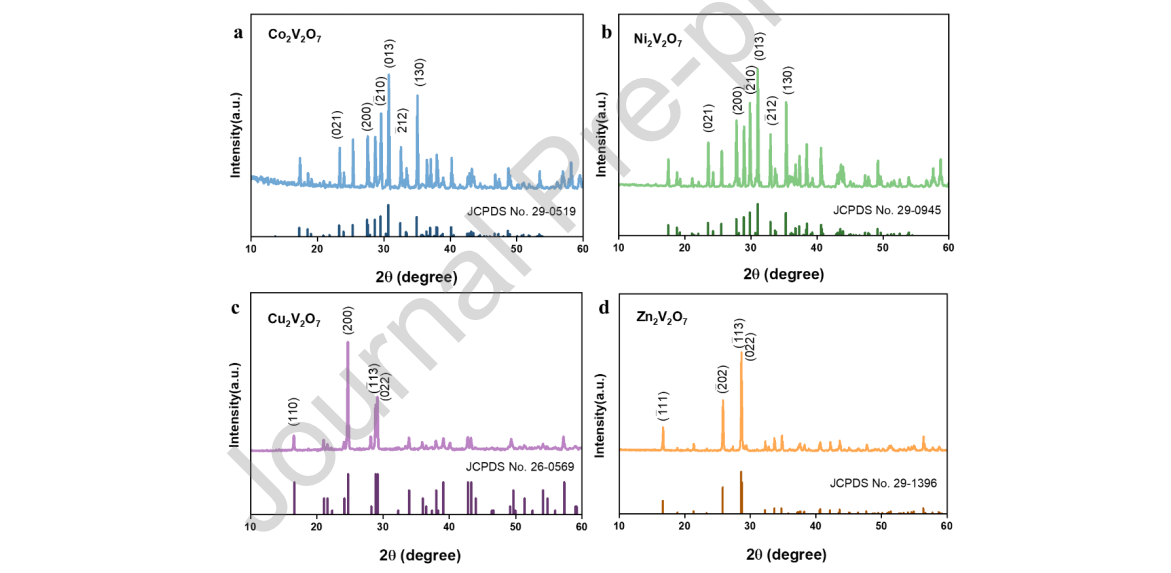
Figure 1. XRD patterns of (a) Co2V2O7, (b) Ni2V2O7, (c) Cu2V2O7 and (d) Zn2V2O7.
To visualize the coordination number associated with the structure, crystal structures of monoclinic vanadate systems with polyhedral representation were drawn using VESTA software. As shown in Figure 2a and 2b, Ni2V2O7 and Co2V2O7 had a similar structure, in which V2O7 anions occur in an eclipsed conformation with a V–O–V angle of ~117.6°, indicating that they belong to the dichromate structures. [21] One of the most remarkable structural features was that magnetic Ni2+ or Co2+ ions had two crystallographic sites with arrays of edge-shared NiO6 or CoO6 octahedra forming skew chains along the c-axis, and the skew chains were separated by nonmagnetic bitetrahedral V2O7, resulting in a quasi-1D structural arrangement. [22] Zn2V2O7 and Cu2V2O7 were isostructural with a space group of C2/c, as shown in Figure 2c and 2d, with a V–O–V angle of ~134.3°. One of the most remarkable structural features was that all Cu2+ or Zn2+ were equivalent to arrays of edge-shared CuO5 or ZnO5 polyhedrons forming linear chains, and the linear chains were not parallel to the same direction but extended in two approximately perpendicular directions, showing a cross-linking chain framework. [23] 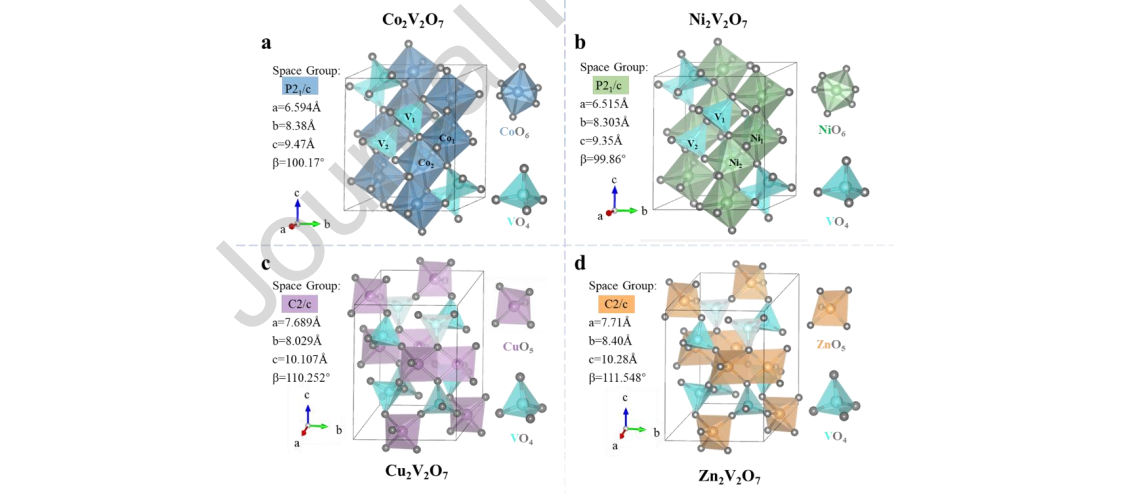
Figure 2. Schematic crystal structure and lattice constant of (a) Co2V2O7, (b) Ni2V2O7, (c) Cu2V2O7 and (d) Zn2V2O7.
The morphology and crystal structure were studied by scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM). As shown in Figure S1-S4, the as-obtained samples were composed of irregular particles of approximately 3 μm, and elemental mapping images further revealed the uniform distribution of elemental A, V, and O on the surface of the corresponding samples. The HRTEM image in Figure 3 revealed that the interplanar spacings of 2.09 Å, 2.88 Å, 3.14 Å, and 2.61 Å were relevant to Co2V2O7 (0 4 0) facets, Ni2V2O7 (0 1 3) facets, Cu2V2O7 (1 1 2) facets, and Zn2V2O7 (2 2 0) facets, respectively. The insets in Figure 3 show the FFT pattern taken on all the samples, which again confirmed the crystalline nature of all the samples. The BET specific surface areas were measured by nitrogen adsorption at 77 K, as displayed in Figure S5. Their specific surface areas of the samples were similar, which was consistent with the particle sizes observed by SEM, and the influence on the catalytic performance can be ignored. X-ray photoelectron spectroscopy (XPS) was also carried out (Figure S6-S9) to understand the chemical composition and the chemical state of metals and oxygen on the surface of A2V2O7 (A = Co, Ni, Cu and Zn), and the result collectively further confirmed that the as-prepared material was the sample we envisioned without any impurities.
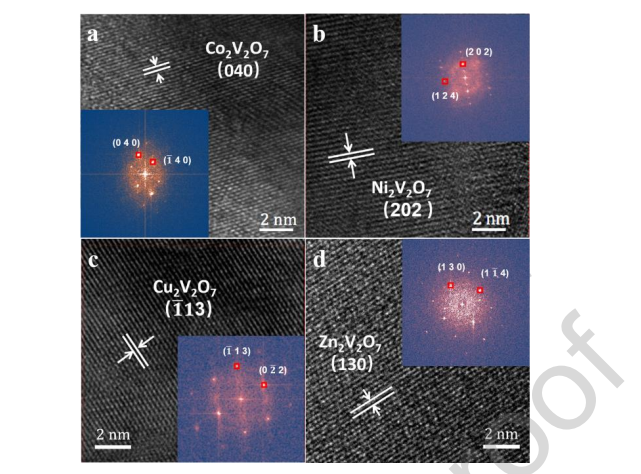
Figure 3. HR-TEM images of (a) Co2V2O7, (b) Ni2V2O7, (c) Cu2V2O7 and (d)
Zn2V2O7. (Inset: Diffraction pattern after Fourier transform)
3.2 Optical properties of A2V2O7 (A = Co, Ni, Cu, Zn)
The optical properties of the synthesized samples were investigated using diffuse reflection ranging from ultraviolet to the near-infrared region, including the visible-light region, and then transformed into the absorption spectra according to the Kubelka-Munk relationship. As shown in Figure 4a, A2V2O7 (A = Co, Ni, Cu, Zn) held a band gap that was able to absorb visible light, implying the possibility of photocatalytic activity. This ability to absorb visible light is due to the d-d transition of A2+. Zn2V2O7 possesses a d10 electronic configuration, with the absence of a d-d transition. The absorption peak indicated an intrinsic absorption assigned to the metal ligand charge transfer. [24, 25] The main absorption edges were ∼420 nm for Zn2V2O7, ∼560 nm for Ni2V2O7 (3A2g → 3T1g(G)), ∼630 nm for Cu2V2O7 (2B1g → 2Eg, 2B1g → 2A1g) and ∼760 nm for Co2V2O7 (T1g → 4T1g(P), 4T1g → 4A2g) in the visible-light region. In the near infrared spectra (Figure S10), two absorption peaks at ~715 nm and ~1,400 nm of Ni2V2O7 were preliminarily assigned to 3A2g → 3T1g(F) and 3A2g → 3T2g transitions, respectively. [7] An obvious peak at ~1450 nm of Co2V2O7 was attributed to 4T1g → 4T2g transitions, and the absorption band near 860 nm of Cu2V2O7 was assigned to 2B1g → 2B2g transitions. [26–29] The d-d transition of the transition metal resulted in abundant optical properties of A2V2O7 pyrovanadates.
Tauc plots based on the optical absorption spectra, XPS valence band spectra (Figure 4c) and Mott-Schottky plots (Figure 4d) were plotted to investigate the band structure of the prepared bimetallic vanadium oxides. According to the electronic bandgap structures of the samples displayed in Figures S11-S14, all adopt indirect electronic transmission between the CBM and VBM. [30]
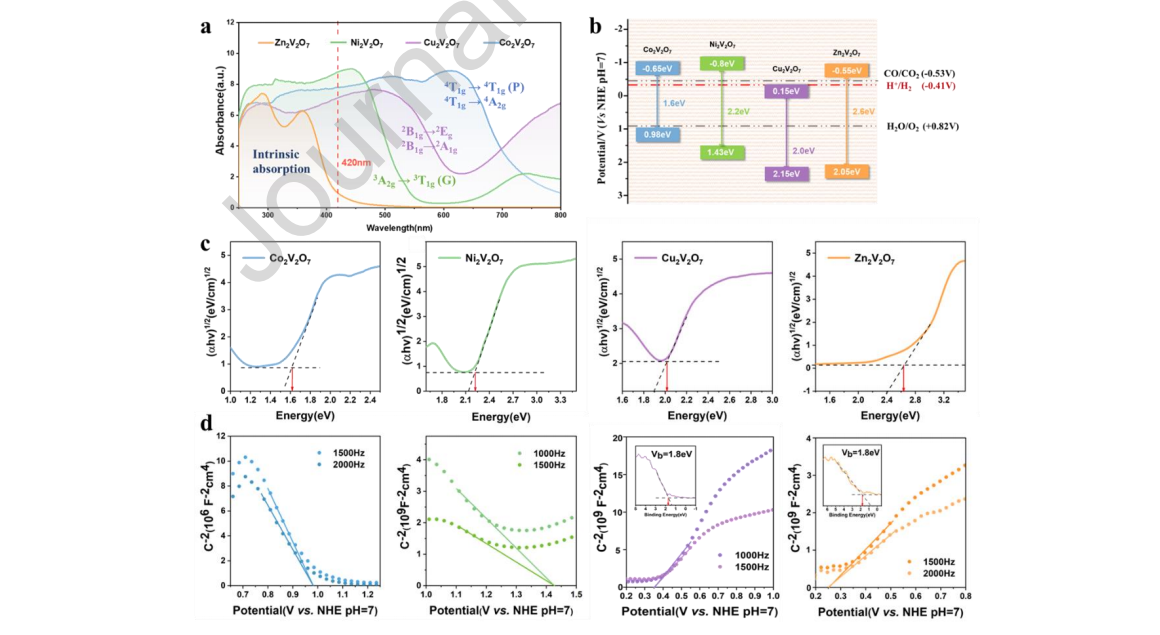
Figure 4. (a) UV–Vis absorption spectrum from 250 nm to 800 nm, (b) Schematic diagram of the estimated band positions of vanadate A2V2O7 (A = Co, Ni, Cu, Zn), (c) Tauc plots and (d) Mott-Schottky plots of vanadate A2V2O7 samples. Insets in figure (d) are the XPS valence band spectrum of the corresponding sample. (Blue: Co2V2O7, green: Ni2V2O7, purple: Cu2V2O7, yellow: Zn2V2O7.)
Given that, the bandgaps were calculated by Tauc plots as 1.62 eV, 2.23 eV, 2.02 eV and 2.62 eV for A2V2O7 (A= Co, Ni, Cu, Zn), respectively, which indicated good agreement with the absorption edges. Mott-Schottky plots were extracted at 1000, 1500, and 2000 Hz separately. The positive slope of the plot confirmed the n-type semiconductor of Cu2V2O7 and Zn2V2O7, while the negative slope indicated the p-type conductivity behavior of Co2V2O7 and Ni2V2O7. In addition, the flat band potential (vs. NHE) was determined to be 0.98 V, 1.43 V, 0.35 V and 0.25 V, respectively, for A2V2O7 (A= Co, Ni, Cu, Zn). The Efb position of p-type semiconductors could be approximately equal to the upper edge of the VB. [31] Therefore, the VB positions of Co2V2O7 and Ni2V2O7 were 0.98 eV and 1.43 eV, and the CB positions were –0.65 eV and –0.8 eV, respectively. The VB position of the n-type semiconductor must be obtained by subtracting the valence band energy from the Efb position. [32] The XPS valence bands of Cu2V2O7 and Zn2V2O7 were all 1.8 eV (insets in Figure 4d). Therefore, their VB positions were 2.15 eV and 2.05 eV, and their CB positions were 0.15 eV and –0.55 eV, respectively.
To facilitate comparison, the band structure of bimetallic vanadium oxides relative to the redox potentials of CO2 and H2O versus NHE at pH = 7 was drawn, as shown in Figure 4b. An eligible photocatalyst that can reduce CO2 with H2O as a reducing agent under illumination needs to provide the potential negative enough to reduce CO2 into value-added chemical fuels (such as CO, CH4, CH3OH and HCHO) and positive enough to oxide water. [33] Obviously, Co2V2O7, Ni2V2O7 and Zn2V2O7 were theoretically able to reduce CO2, but the CB potential of Cu2V2O7 was far from the CO2 reduction potential. Therefore, Cu2V2O7 did not have the ability of photocatalytic CO2 reduction.
From the above experimental results, the change in the A-site will affect the light absorption characteristics, band gap width, valence band and conduction band position of the samples. These factors are important properties affecting the photocatalytic ability of samples.
3.3 Electronic structure analysis of A2V2O7 (A = Co, Ni, Cu, Zn)
Information about the band structure is important for understanding the photocatalytic reaction. We calculated the band structures of A2V2O7 with the plane-wave-based density functional method, which is widely used to predict the band structures of semiconductors. The results are shown in Figure 5. Among these wavefunctions, the CB composition is very similar and mainly contrasted with the V 3d and O 2p orbitals. These bands have antibonding V 3d-O 2p orbital character derived from the VO4 tetrahedron. For Ni2V2O7 (or Co2V2O7), few Ni 3d (or Co 3d) orbitals were introduced in the CBs because the oxygen atom coshared in V2O7 was connected to the NiO6 (or CoO6) octahedra. This connection did not appear in Cu2V2O7 or Zn2V2O7. However, the VB composition is very different. In particular, the VBs of the four compounds mainly result from the intense interaction between the O 2p and A 3d (A = Co, Ni, Cu, Zn) orbitals, coming from the AOx (A = Co, Ni, Cu, Zn, x = 5 or 6) polyhedron, which improved the valence band maximum to a narrow band gap, leading to their visible-light response.
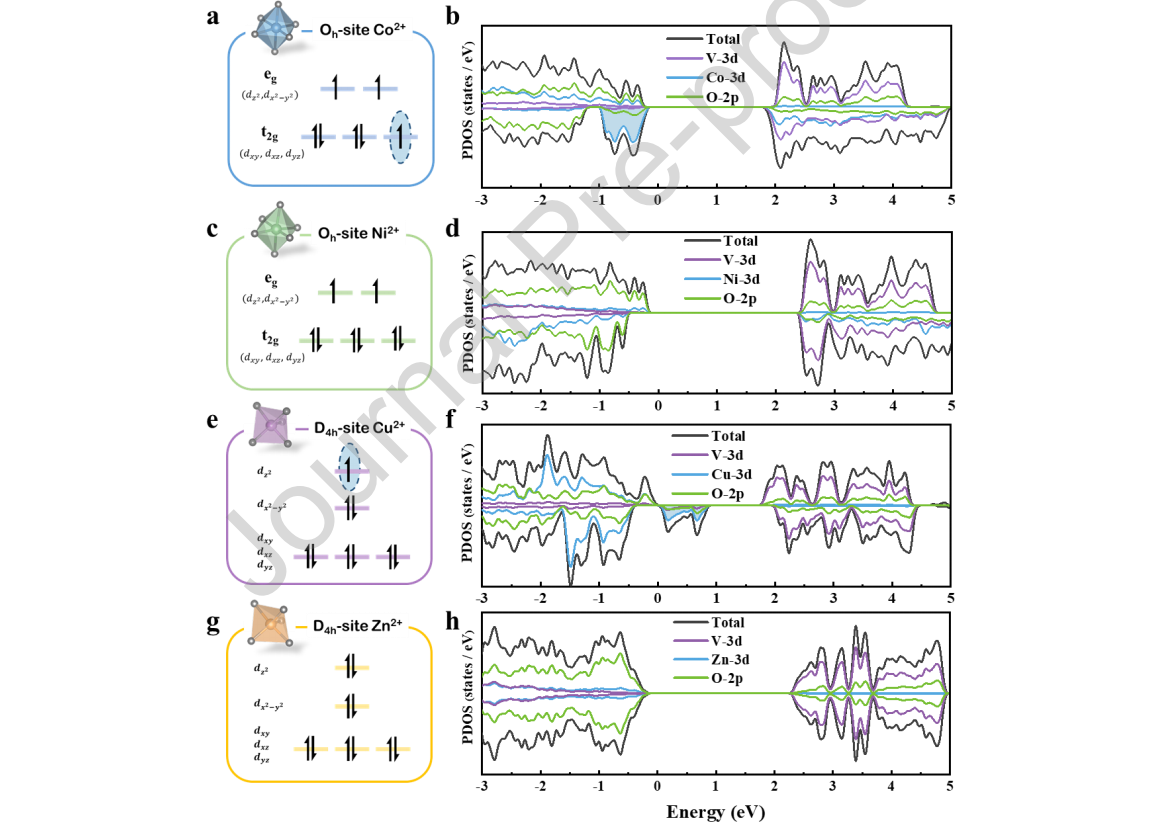
Figure 5. The 3d electron configuration of (a) Co2+, (c) Ni2+, (e) Cu2+ and (g) Zn2+ ions in vanadate. Projected total density of states (DOS) for (b) Co2V2O7, (d) Ni2V2O7, (f) Cu2V2O7, and (h) Zn2V2O7.
It is obvious that there is a nearly isolated band energy at the Fermi level in the PDOS of Cu2V2O7, which is a feature of Cu2+ when combined with O atoms. [33] Among these outer-shell electrons (Figure 5e), the unpaired electron usually exhibited a more positive energy. [34] This metallicity of Cu2V2O7 was also reflected in the electronic band structure, as shown in Figure S13, and the phenomenon likely pulled the CBM downward to a position that was not enough to reduce CO2, along with narrowing the band gap. The PDOS result was consistent with the above experimental result that Cu2V2O7 does not meet the thermodynamic requirement for CO2 reduction. Thus, we would not discuss its photocatalytic ability and electronic structure subsequently.
Table 1. Physical property and photocatalytic activities of A2V2O7 (A = Co, Ni, Zn).
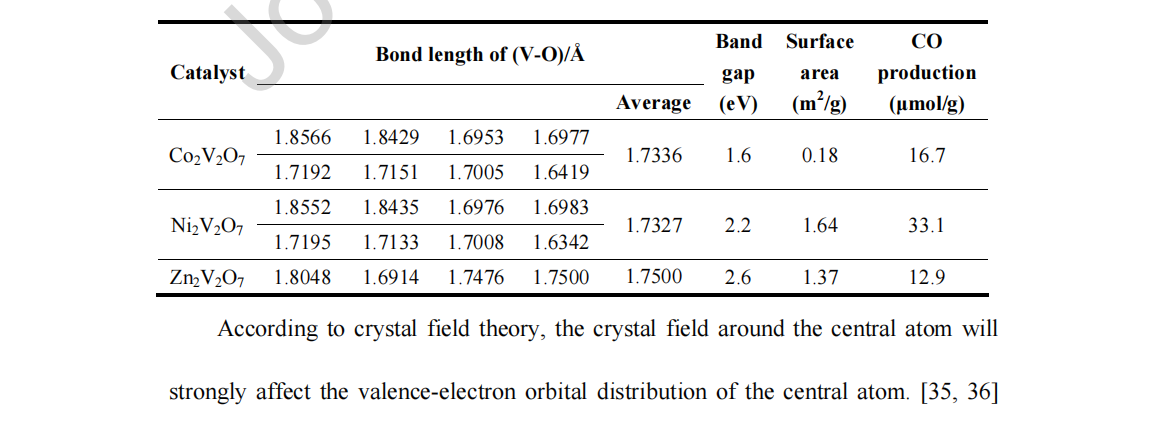 For A2V2O7 (A = Co, Ni, Zn), which all contain VO4 tetrahedra in their crystal structures, the shorter the bond length of V–O is, the stronger the effect of the surrounding crystal field, and the greater the orbital of V 3d splits. [37] From the crystal structures of these V-based materials, the average V–O distance was calculated to be 1.733 Å in Ni2V2O7, 1.734 Å in Co2V2O7 and 1.750 Å in Zn2V2O7, as shown in Table 1. This meant that the V–O distance in Ni2V2O7 was less than that in Co2V2O7, which was less than that in Zn2V2O7. This indicated that Ni2V2O7 had the strongest crystal field and a greater splitting of the V 3d, which resulted in the widest V 3d band distribution. This finding was evident in the total electronic bandgap structures of the photocatalysts (Figure S15). The greater electronic state distribution width represented a larger dispersion in κ-space, which indicated that the photogenerated electrons in the Ni2V2O7 sample might possess the smallest effective mass among the three metals, vanadate A2V2O7 (A = Co, Ni, Zn). [38, 39] The charge separation and transfer behaviors of Ni2V2O7, Co2V2O7 and Zn2V2O7 have been investigated by photoelectrochemical measurements. The linear sweep voltammetry (LSV) curves demonstrated that all of them were photocatalysts. The corresponding transient photocurrent I-t curves are shown in Figures S18 and S19. The photocurrent density of Ni2V2O7 was obviously larger than that of the Co2V2O7 sample. The photocurrent density of Zn2V2O7 was much smaller than both. This result indicated that more efficient separation and migration of photogenerated electron-hole pairs occurred on the Ni2V2O7 sample. Therefore, among them, Ni2V2O7 would have the highest photocatalytic activity for CO2 reduction.
For A2V2O7 (A = Co, Ni, Zn), which all contain VO4 tetrahedra in their crystal structures, the shorter the bond length of V–O is, the stronger the effect of the surrounding crystal field, and the greater the orbital of V 3d splits. [37] From the crystal structures of these V-based materials, the average V–O distance was calculated to be 1.733 Å in Ni2V2O7, 1.734 Å in Co2V2O7 and 1.750 Å in Zn2V2O7, as shown in Table 1. This meant that the V–O distance in Ni2V2O7 was less than that in Co2V2O7, which was less than that in Zn2V2O7. This indicated that Ni2V2O7 had the strongest crystal field and a greater splitting of the V 3d, which resulted in the widest V 3d band distribution. This finding was evident in the total electronic bandgap structures of the photocatalysts (Figure S15). The greater electronic state distribution width represented a larger dispersion in κ-space, which indicated that the photogenerated electrons in the Ni2V2O7 sample might possess the smallest effective mass among the three metals, vanadate A2V2O7 (A = Co, Ni, Zn). [38, 39] The charge separation and transfer behaviors of Ni2V2O7, Co2V2O7 and Zn2V2O7 have been investigated by photoelectrochemical measurements. The linear sweep voltammetry (LSV) curves demonstrated that all of them were photocatalysts. The corresponding transient photocurrent I-t curves are shown in Figures S18 and S19. The photocurrent density of Ni2V2O7 was obviously larger than that of the Co2V2O7 sample. The photocurrent density of Zn2V2O7 was much smaller than both. This result indicated that more efficient separation and migration of photogenerated electron-hole pairs occurred on the Ni2V2O7 sample. Therefore, among them, Ni2V2O7 would have the highest photocatalytic activity for CO2 reduction.
3.4 Photocatalytic activities of A2V2O7 (A = Co, Ni, Zn)
To verify the results of the theoretical calculation, photocatalytic CO2 reduction tests were carried out over the as-prepared vanadate samples under visible light irradiation (λ ≥ 420 nm) using H2O as a reducing agent. The typical time-course curves of photocatalytic CO2 reduction are shown in Figure 6a.
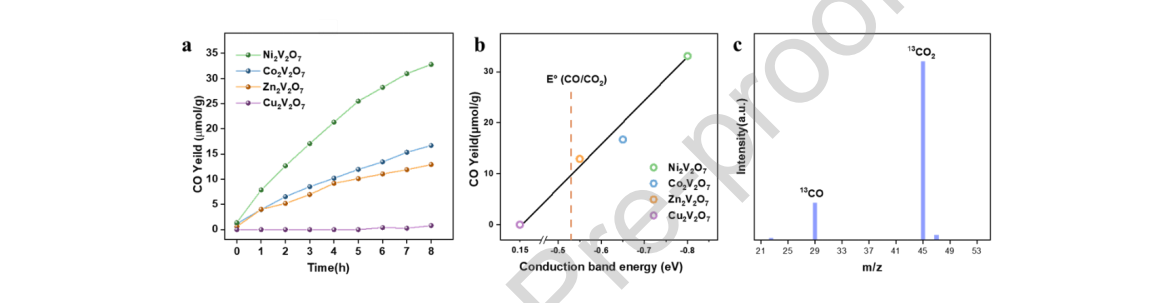
Figure 6. (a) CO evolution by CO2 reduction as a function of irradiation time over four vanadate A2V2O7 (A = Co, Ni, Cu, Zn) under a 300 Xe lamp (λ ≥ 420 nm). Specific values are shown in Table 1. (b) Correlation between the yield of CO and the conduction bands of semiconductor catalysts. The dashed line denotes the standard redox potential of a CO/CO2 couple. (c) GC-MS spectrum of the products after 13CO2 reduction.
As we expected, Cu2V2O7 was inactive to reduce CO2 under continuous light irradiation owing to its positive conduction band position. For Co2V2O7, Ni2V2O7 and Zn2V2O7, CO was produced from CO2 under visible light irradiation, and their CO yields increased with prolonged irradiation time. Among the four as-prepared metal vanadates, Ni2V2O7 exhibited the highest activity for photocatalytic CO2 reduction under visible light, and the yield of CO achieved 33.1 μmol/g after 8 h. The yields of CO over Co2V2O7 and Zn2V2O7 reached 16.7 and 12.9 μmol/g, respectively. This result was consistent with the predicted result that a smaller effective mass for the sample might give rise to higher photocatalytic activity for CO2 reduction. Interestingly, from the perspective of crystal structure, materials of the P21/c space group as a whole had better properties than the C2/c space group, which may be caused by the different symmetries of the two space groups. [40] Co2V2O7 and Ni2V2O7 had a more distorted space group, which facilitated charge separation and contributed to an increase in the photocatalytic activity. [41] In addition, we have found that the order of CO yield over three pyrovanadates A2V2O7 (A = Co, Ni, Zn) was consistent with that of the reduction potential of the CBM, which was in accordance with the previous report that the yields of products from CO2 reduction increased as the CBM becomes more negative than the redox potential of a certain reaction of CO2 reduction by Fujijima et al. (Figure 6b). [42]
To evaluate the stability of the Ni2V2O7 sample, repeated photocatalytic experiments were carried out, and the results are displayed in Figure S16. After three cycling measurements, the CO yield decreased slightly, remaining approximately 85% of the original, and the crystal structure did not change significantly (Figure S19 – S21). From Table S2, the comprehensive performance of Ni2V2O7 was higher than that of most single component catalysts under visible-light irradiation (λ ≥ 420 nm),which provided a basis for the subsequent modification and performance improvement. When the control experiments were carried out in the dark or without semiconductor catalysts, no reduced products of carbon dioxide were detected, confirming that the above phenomenon results from the photocatalytic reduction process. Furthermore, 13C-labeled isotope labeling experiments were carried out to clarify the origin of carbon in the photocatalytic reaction. The signals at m/z = 29 and m/z = 45 detected by GC–MS correspond to 13CO and 13CO2, respectively, proving that the produced CO originated from CO2 rather than other residual organics during the synthetic process, as shown in Figure 6c.
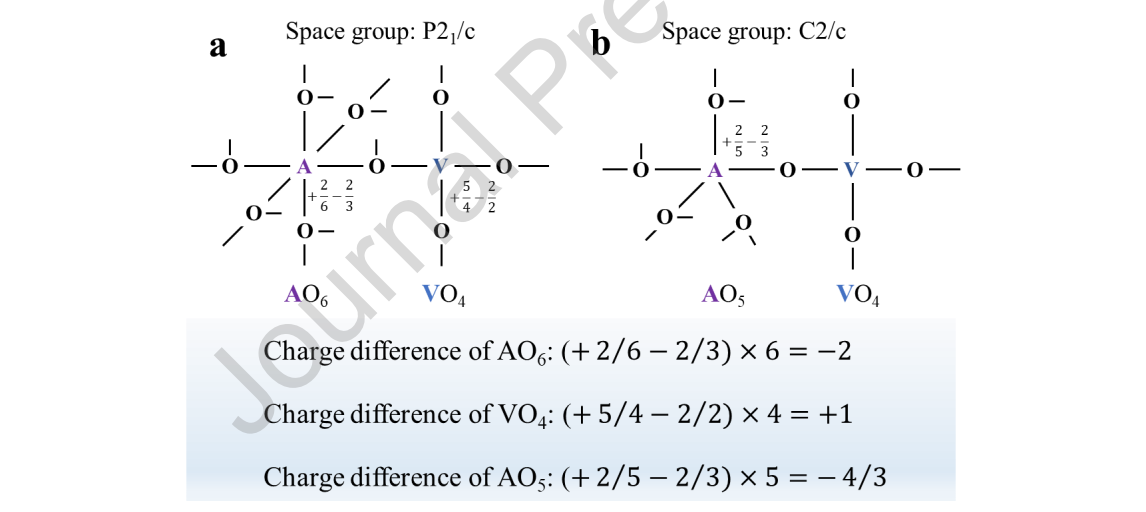
Figure 7. Tanabe hypothesis structure of the (a) P21/c and (b) C2/c space groups. (The calculation process of NiO6, for example, was that two positive charges of Ni2+ were distributed to six bonds, while the two negative charges of oxygen atoms were
distributed to three bonds. The difference in charge for one bond was +2/6 − 2/3, and for all the bonds, the valence unit was (+2/6 − 2/3) × 6 = −2.)
From the abovementioned results, it can be obtained that the substitution of A-site cations changed the electron configuration and band structure thermodynamically. Meanwhile, this change might affect the catalytic properties of the samples in terms of kinetics. Binary metal oxides are well known to have surface acidity and basicity, which are affected by the coordination numbers and valences of metal atoms. [43] According to the Tanabe hypothesis, an excess of a negative or positive charge of a binary oxide can be simulated in a model structure. [44] In Figure 7, we constructed and calculated the charge difference diagram of A2V2O7. Whether the space group is P21/c or C2/c, divalent transition metal A was surrounded by excess negative charges, which easily gave electrons and showed the characteristics of basic sites. Therefore, we speculated that the A site was likely to be the active center of the reaction
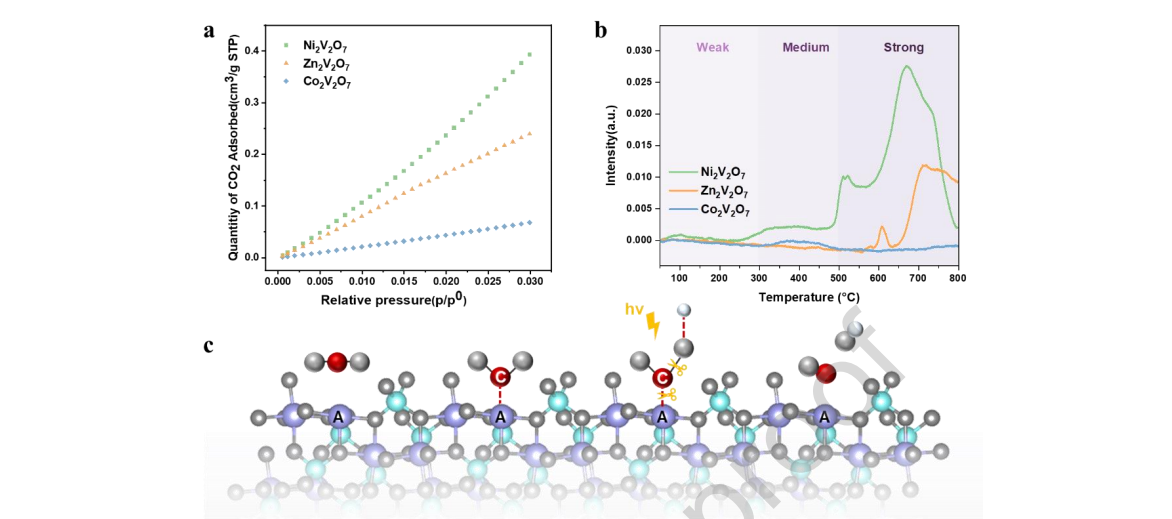
Figure 8. (a) CO2 adsorption isotherm, (b) CO2-TPD profiles of A2V2O7 (A = Co, Ni, Zn) samples. (c) The possible reaction mechanism of CO2 photoreduction on A2V2O7 samples.
Subsequently, CO2 adsorption and temperature-programmed (TPD) experiments were carried out to investigate the adsorption properties and surface basicity of Co2V2O7, Ni2V2O7 and Zn2V2O7. As shown in Figure 8a, Ni2V2O7 had a higher CO2 adsorption capacity, which was beneficial to the first step of photocatalytic CO2 reduction. The CO2-TPD results (Figure 8b, Table S1) further confirmed that when nickel ions were located at the A site, the vanadate surface was loaded with more basic sites. Usually, the types of basic sites in the reduced catalysts were divided into three temperature regions, i.e., physical adsorption of CO2 (< 300 °C), HCO3− or HCO2− (300 °C - 500 °C), and carbonates (CO3 2−) (500 °C - 800 °C), which were attributed to weak, medium, and strong basic sites of catalysts, respectively. [45, 46]
The medium basic sites among them were the most likely to be active sites for CO2 reduction because they had a balanced character between reactant adsorption and product desorption. [47]
Based on the above assumptions and experiments, we can reasonably conclude that the adsorption and activation of CO2 are more likely to occur at the A-site. Therefore, a possible reaction path at the vanadate surface is proposed, as shown in Figure 8c. First, the CO2 molecule was adsorbed on the A atoms through hybridization between the 2p and 3d orbitals of A to C, and the adsorbed carboxylate CO2− species was formed on A sites. [48] Second, carboxylate was protonated to produce HCO2− , and under light irradiation, the photogenerated electron transferred to the C–O bond of carboxylate. [49] Finally, the above interaction promoted the breakage of the C–O bond of the adsorbed carboxylate species, and thus, the CO molecule was generated. Therefore, the role of A was of great significance, leading to improved surface basic sites, facilitating the adsorption of CO2 as well as the formation of a series of reactive intermediates.
4. Conclusions
We synthesized a series of metal vanadates, A2V2O7 (A = Co, Ni, Cu, Zn), to probe the A cation-reactivity relationship for CO2 reduction under visible-light irradiation. Theoretically calculated energy band structures revealed that the A site ions played an important role in the electronic structure and seriously influenced the photocatalytic properties of A2V2O7. For Cu2V2O7, its conduction band edge was too positive to reduce CO2. The other three groups of A2V2O7 (A = Co, Ni, and Zn) had suitable energy to reduce CO2. Among them, Ni2V2O7 had a greater splitting of V 3d, resulting in the widest conduction band distribution, which indicated the largest mobility of photogenerated electrons. Moreover, the experimental results showed that Ni2V2O7 had the most negative conduction band, resulting in better CO2 reducibility. In addition, the Tanabe hypothesis predicted that A cations with surface basicity were the active sites of the photocatalytic CO2 reduction. The CO2-TPD result revealed that Ni2+ at the A site brought the strongest surface basicity. All of these factors led to the highest photocatalytic activity for CO production on Ni2V2O7 under visible light. The present work contributes to deeply understanding and designing metal vanadate-containing photocatalysts with high photocatalytic activity for CO2 reduction.
CRediT authorship contribution statement
Mengyang Du, Yong Chen and Zhaosheng Li conceived and designed the idea; Mengyang Du conducted the sample synthesis and characterization and prepared the manuscript; Xiaoming Xu and Yuanming Zhang conducted the photoelectrochemical measurements; Wenjing Wang conducted the theoretical calculation; Yang Li verified the results of the photocatalytic activity; and Zhaosheng Li supervised the experiment and revised the manuscript. The manuscript was written through contributions of all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the National Science Fund for Distinguished Young Scholars (No. 22025202), National Key Research and Development Program of China (Nos. 2018YFA0209303 and 2020YFA0710302), National Natural Science Foundation of China (No. 51972165), and Natural Science Foundation of Jiangsu Province (No. BK20202003) for financial support. The numerical calculations in this study were conducted thanks to the access to the IBM Blade cluster system at the High Performance Computing Center (HPCC) of Nanjing University.
...