Service hotline
+86 18518316054
 Current location : Home page > Resources > Papers > T-shaped and H-shaped polymers constructed from UV-induced strain promoted azide-alkyne cycloaddition reaction
Current location : Home page > Resources > Papers > T-shaped and H-shaped polymers constructed from UV-induced strain promoted azide-alkyne cycloaddition reaction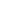
Abstract
Topological polymers with T-shaped and H-shaped molecular architecture were built on the combination of atom transfer radical polymerization (ATRP) and UV-induced strain promoted azide-alkyne cycloaddition (SPAAC) reaction. In the presence of a cyclopropenone-masked dibenzocyclooctyne functionalized dibromo ATRP initiator, reactive polystyrene (PS) was synthesized to have a cyclopropenone-masked dibenzocyclooctyne group in the middle of polymer chain. After releasing dibenzocyclooctyne from deprotecting cyclopropenone-masked dibenzocyclooctyne under UV irradiation, T-shaped and H-shaped topological polymers were constructed by reacting PS with mono-end and di-end azide functionalized poly(ethylene oxide) (PEO), respectively, based on the SPAAC reaction.
Introduction
Polymer topology plays an important role in determining the properties and functionalities of polymer materials.1 The synthesis of topological polymers has been always a core direction in the field of polymer chemistry. Thanks to the development of modern living/quasiliving polymerization2 techniques (LP) and the wide application of click chemistry in polymer science, a variety of polymer topology has been fabricated based on their combination including starlike,3 brushlike,4 grafted,5 hyperbranched,6 cyclic macromolecular7 architectures and polymer networks.8 In this strategy, LP was used to prepare varied polymer building blocks functionalized with clickable groups. The click reactions were then used to couple the related building blocks to form the topological polymers. As the simplest branched polymers, synthesis of T-shaped (3-arm star) and H-shaped polymers could be viewed as the golden candidate to evaluate the combination of LP and click chemistry for fabricating polymer topology.
To date, a series of LP and click chemistry combined methods have been developed for the formation of T-shaped and H-shaped polymers. Based on the LP, a series of click reactions have been used to fabricate the T-shaped and H-shaped polymers, including copper-catalyzed azide-alkyne cycloaddition (CuAAC),3,9-13 Diels-Alder reaction,14,15 and thiol-ene reaction.16,17 In these techniques, CuAAC was one of most important click reactions due to its high efficiency and small sized reaction groups. In addition, the azide group could be easily introduced at the end of polymers based on atom transfer radical polymerization (ATRP) and substitution of bromide end group by azide. To achieve high reaction efficiency, however, a large amount of copper catalysts was required by CuAAC. This may cause the contamination of the resultant topological polymers and restrict their application in the field of advanced technologies such as biology and photoelectronics.
Recently, the strain promoted azide-alkyne cycloaddition (SPAAC) reaction was developed as a bio-orthogonal reaction to circumvent the awkward situation of CuAAC in biology.18 By confining an alkyne in a small ring molecule, the strain activated alkyne group could efficiently react with azide group at room temperature requiring no any catalyst. Since its birth, SPAAC has been widely used in the formation of bio-conjugates in vitro and in vivo.19 In addition, SPAAC has also been applied to prepare polymeric materials including polymer surface brush20 and networks.21 Considering the importance of SPAAC click reaction, we synthesized a dibenzocyclooctyne functionalized ATRP initiator and systematically studied its ATRP behavior.22 For polymerizing styrenic and methacrylate monomers, the polymerization held living characteristics. For polymerizing acrylate monomers, however, the polymerization lost living behavior. Based on the combination of ATRP and SPAAC, we successfully prepared the block, star and brush topological polymers. 22,23 Furthermore, by designing the ATRP initiator containing a cyclopropenone-masked dibenzocyclooctyne, the well-defined polystyrenics, polymethacrylates and polyacrylates could be produced by a standard ATRP protocol and the polymerizations all behaved living characteristics.24 Under UV irradiation, cyclopropenone-masked dibenzocyclooctyne quantitatively released the reactive dibenzocyclooctyne group, which in-situ reacted with azide by the SPAAC mechanism. By the combinations of ATRP/UV-induced SPAAC25 and macromolecular design via the interchange of xanthate (MADIX)/UV-induced SPAAC,26 the cyclic polymer topology has been successfully prepared.
As a continuous contribution in this research topic herein, T-shaped and H-shaped polymers were prepared by the combination of ATRP and UV-induced SPAAC click reaction. As shown in Scheme 1, by a difunctional ATRP initiator, the well-defined linear PS building blocks were synthesized from ATRP to have a cyclopropenone-masked dibenzocyclooctyne in the middle of the polymer chain. After quantitatively releasing dibenzocyclooctyne group under UV irradiation, T-shaped and H-shaped hybrid copolymers were prepared by reacting linear PS building blocks with mono- and di-end azide functionalized poly(ethylene oxide) (PEO), respectively.
2. Experimental section
Styrene (St, 99%, Aldrich) was dried over CaH2 and distilled under reduced pressure. Copper (I) bromide (CuBr, 98%, J&K) was washed by acetic acid and ethanol. Tetrahydrofuran (THF, AR, Beijing Chemical Reagent Co.) was distilled from sodium/benzophenone. Cyclopropenone-masked dibenzocyclooctyne functionalized ATRP initiator,24 mono- and di-end azide functionalized PEO22 (PEO23-N3 and N3-PEO23-N3) were synthesized according to the literatures. Pentamethyldiethylenetriamine (PMDETA, 99%, Energy Chemical), anisole (AR, Beijing Chemical Reagent Co.) and other reagents were purchased from commercial retailers and used as received. A low pressure mercury lamp (120 W) (CEL-LPH120-254, Beijing China Education Au-light co.Ltd) was used as the UV light source.
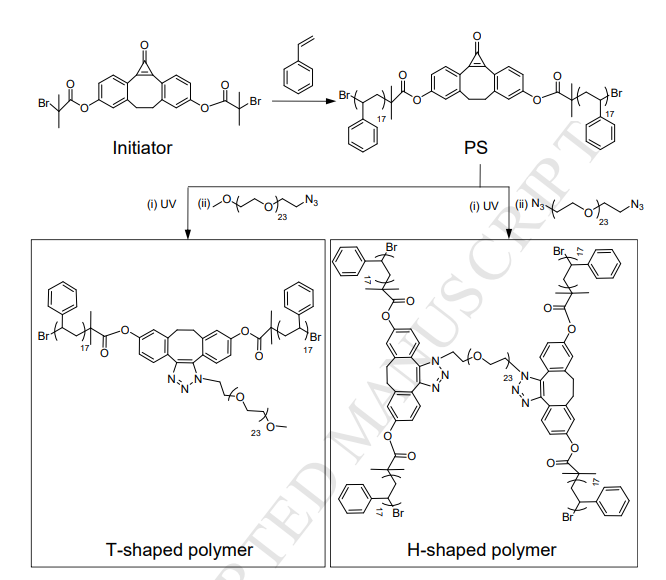
Fig. 1. 1H NMR spectrum of cyclopropenone-masked dibenzocyclooctyne functionalized PS in CDCl3. Fig. 2A (black) shows the GPC curve of linear PS, in which a well-defined monomodal and symmetric peak was observed. The Mn,GPC and Mw/Mn were integrated as 4880 and 1.04, respectively. It is reported that cyclopropenone-masked dibenzocyclooctyne ATRP initiator possessed a characteristic peak at 314 nm in the UV-Vis spectrum.24 As shown in Fig. 3A (black), linear PS inherited the peak of 314 nm from the ATRP initiator. These results clearly indicated the successful preparation of linear PS with the cyclopropenone-masked dibenzocyclooctyne group in the middle of polymer chain.
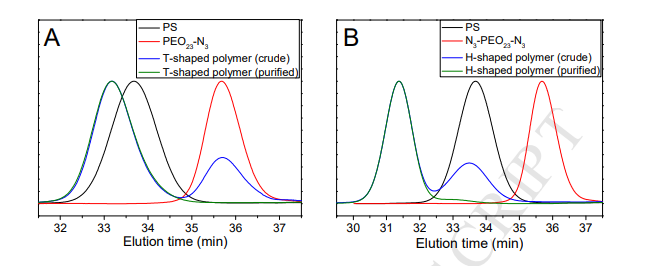
Fig. 2. (A) GPC curves of PS (black), PEO23-N3 (red), crude (blue) and purified (olive) T-shaped polymers. (B) GPC curves of PS (black), N3-PEO23-N3 (red), crude (blue) and purified (olive) H-shaped polymers. THF was used as the eluent and polystyrene standards were used for the calibration.
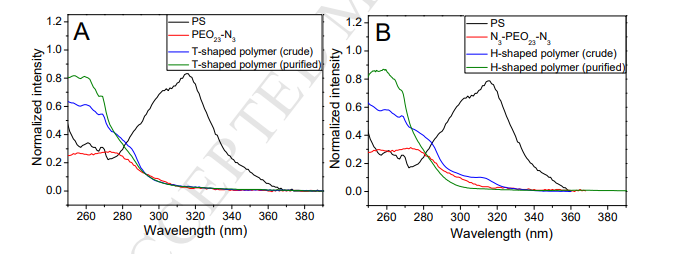
Fig. 3. (A) UV-Vis spectra of PS (black), PEO23-N3 (red), crude (blue) and purified (olive) T-shaped polymers in THF. (B) UV-Vis spectra of PS (black), N3-PEO23-N3 (red), crude (blue) and purified (olive) H-shaped polymers in THF.